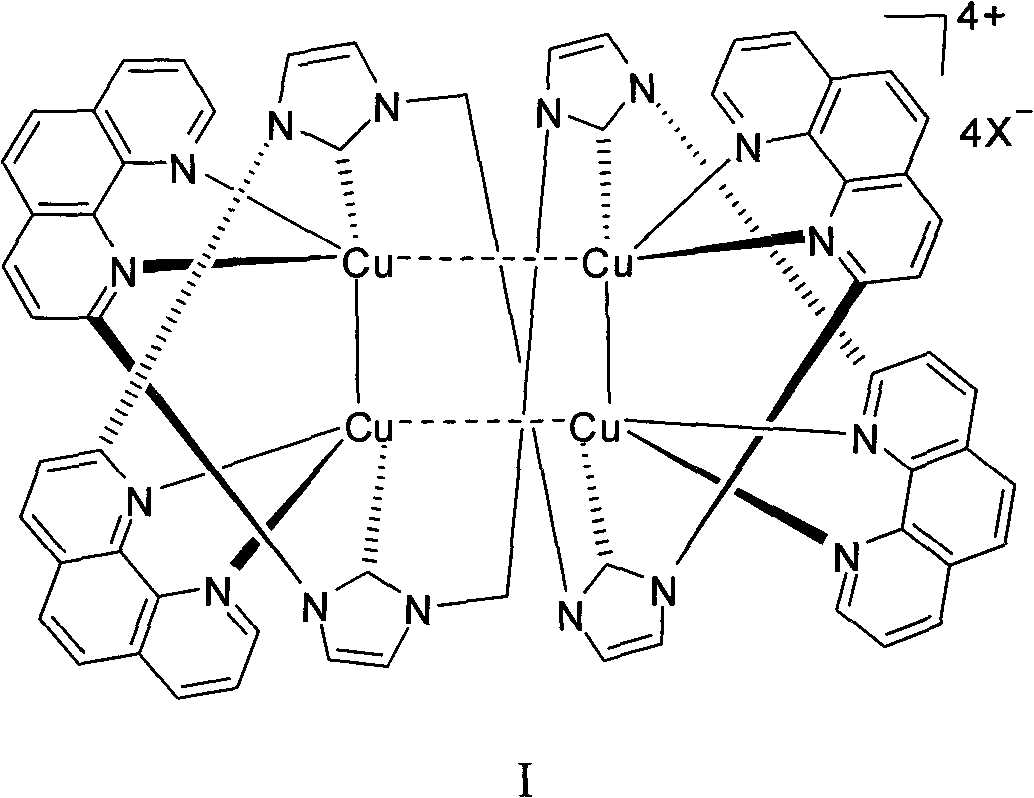
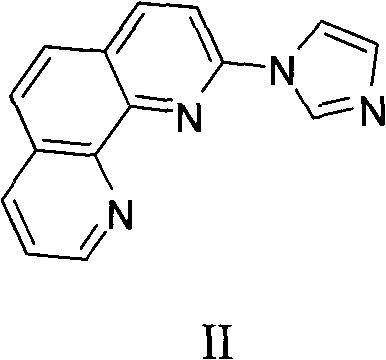
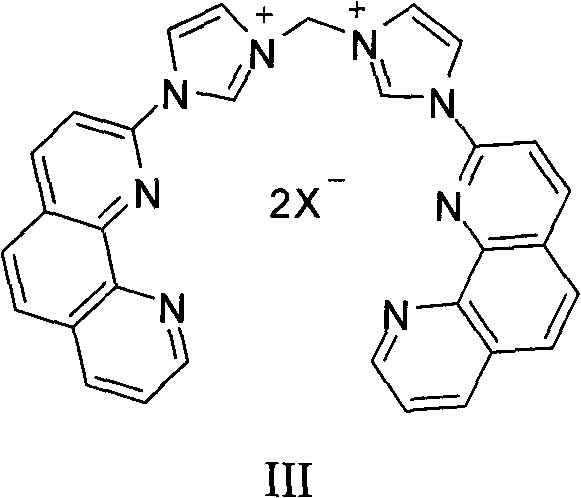
1,10-phenanthroline hydrate functionalized n-heterocyclic carbene and tetranuclear copper compound and preparation method for same
A technology of nitrogen heterocyclic carbene and o-phenanthroline, applied in the field of o-phenanthroline functionalized nitrogen heterocyclic carbene tetranuclear copper compound and its preparation, achieving the effects of convenient operation and control, high yield and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] a Under the protection of nitrogen at 1 standard atmospheric pressure, 2-iodo-o-phenanthroline (3.06g, 10mmol), imidazole (1.02g, 15mmol), cuprous oxide (2.16g, 15mmol), potassium carbonate (2.07g , 15mmol) and 45mLN, N'-dimethylformamide was added into a 100mL Schlenk reaction tube, reacted at 150°C for 15 hours, filtered after cooling to obtain a quality of 1.96g, and the yield was 80%. The molecular structural formula is:
[0027]
[0028] The calculated elemental analysis value of 2-imidazolyl-o-phenanthroline is C, 73.16; H, 4.09; N, 22.75; the measured elemental analysis value of 2-imidazolyl-o-phenanthroline is C, 73.30; H, 4.11; N, 22.45; The NMR spectrum of 2-imidazolyl-phenanthroline is: 1 H NMR (400MHz, CDCl 3 ): δ9.18(d, J=2.0Hz, 1H), 8.63(s, 1H), 8.35(d, J=8.4Hz, 1H), 8.24(d, J=8.0Hz, 1H), 7.99(s , 1H), 7.78(s, 2H), 7.68(d, J=8.0Hz, 2H), 7.66-7.62(m, 1H), 7.25(s, 1H). 13 C NMR (100MHz, CDCl 3 ): δ150.4, 147.9, 145.3, 145.2, 139.5, 136.2, 130.8, 129.4...
Embodiment 2
[0042] a Under the protection of nitrogen at 1 standard atmospheric pressure, 2-iodo-o-phenanthroline (3.06g, 10mmol), imidazole (1.02g, 15mmol), cuprous oxide (2.16g, 15mmol), potassium carbonate (2.07g , 15mmol) and 45mLN, N'-dimethylformamide was added into a 100mL Schlenk reaction tube, reacted at 150°C for 15 hours, filtered after cooling to obtain a quality of 1.96g, and the yield was 80%. The molecular structural formula is:
[0043]
[0044] The calculated elemental analysis value of 2-imidazolyl-o-phenanthroline is C, 73.16; H, 4.09; N, 22.75; the measured elemental analysis value of 2-imidazolyl-o-phenanthroline is C, 73.30; H, 4.11; N, 22.45; The NMR spectrum of 2-imidazolyl-phenanthroline is: 1 H NMR (400MHz, CDCl 3 ): δ9.18(d, J=2.0Hz, 1H), 8.63(s, 1H), 8.35(d, J=8.4Hz, 1H), 8.24(d, J=8.0Hz, 1H), 7.99(s , 1H), 7.78(s, 2H), 7.68(d, J=8.0Hz, 2H), 7.66-7.62(m, 1H), 7.25(s, 1H). 13 C NMR (100MHz, CDCl 3 ): δ150.4, 147.9, 145.3, 145.2, 139.5, 136.2, 130.8, 129.4...
Embodiment 3
[0058] a Under the protection of nitrogen at 1 standard atmospheric pressure, 2-iodo-o-phenanthroline (3.06g, 10mmol), imidazole (1.02g, 15mmol), cuprous oxide (2.16g, 15mmol), potassium carbonate (2.07g , 15mmol) and 45mLN, N'-dimethylformamide was added into a 100mL Schlenk reaction tube, reacted at 150°C for 15 hours, filtered after cooling to obtain a quality of 1.96g, and the yield was 80%. The molecular structural formula is:
[0059]
[0060] 2-imidazolyl-phenanthroline.
[0061] b. The compound (1.23g, 5mmol) and methylene bromide (3.5mL) obtained in step a were added into a 20mL Schlenk reaction tube, reacted at 100°C for 15 hours, collected by filtration after cooling to obtain imidazolium bromide, washed with 50mL of ether, and dried in vacuo. Dissolve the dried imidazolium bromide in 20 mL of methanol, and add 0.55 g of NaBF dropwise to the methanol solution 4 In the saturated aqueous solution of the solution, continue to stir for 60 minutes after the dropwise ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com