Transdermally absorbed medicine comprising adjuvant-containing pharmaceutical microparticle and adjuvant-containing water for skin use
A technology for pharmaceutical excipients and drugs, which is used in medical preparations containing active ingredients, skin diseases, drug combinations, etc., can solve the problems of unstable particles and inability to guarantee the Ostwald ripening of particle suspensions, and achieves The effect of reducing waste and ensuring suspension stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
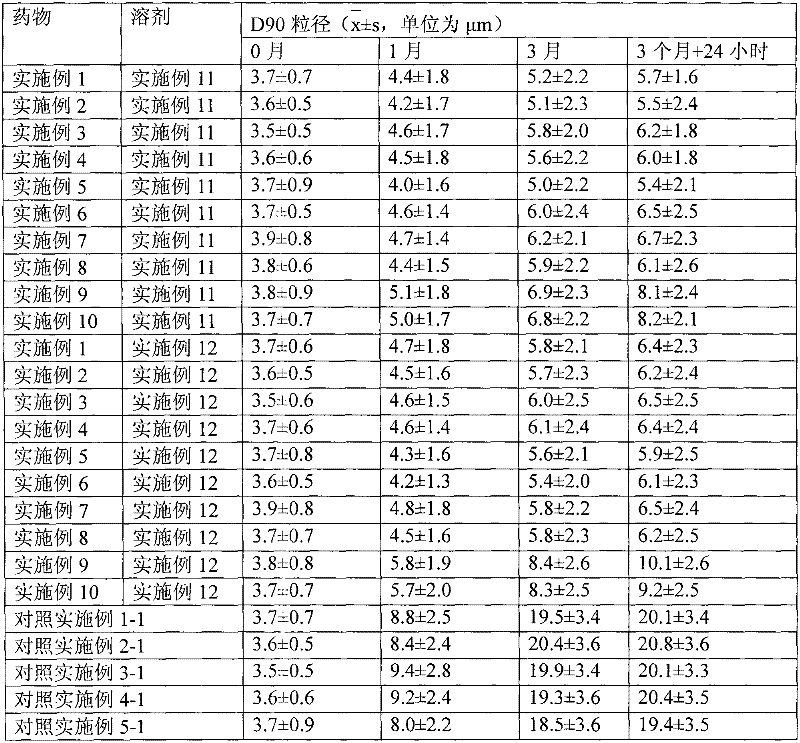
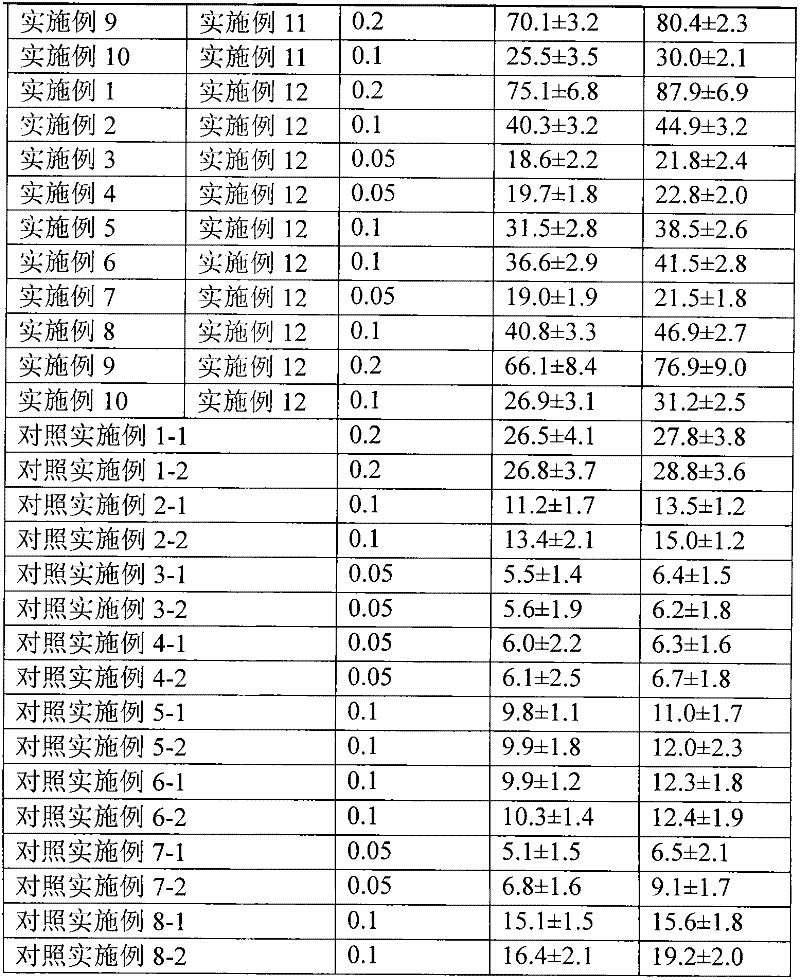
[0040] 1. Preparation of drug particles for transdermal absorption
[0041] The capsules packed with the drug microparticles obtained in Examples 1 to 10 are plant capsules, and the capsules are sealed and packaged with aluminum foil blisters after packing. The water containing auxiliary materials obtained in Examples 11 and 12 is sealed and packed in ampoules.
Embodiment 1
[0043] Dissolve 1g of hydrocortisone in ethanol, filter, sterilize, spray-dry the filtrate, micronize the particle size to 3μm, mix with 1.5g crystalline lactose with a particle size of 30μm, pass through a 200-mesh sieve for 3 times and mix well Finally, it is divided into No. 2 capsules, and each capsule contains 10 mg of hydrocortisone. Electron microscope observation shows that the drug particles are spherical.
[0044] The process conditions are: the inlet temperature is 100°C, the outlet temperature is 70°C, the air flow rate is 90%, the inner diameter of the nozzle outlet is 0.1cm, the air flow rate of the nozzle is 800ml / min, and the injection speed is 50mL / h
Embodiment 2
[0046] Dissolve 1 g of triamcinolone acetonide acetate in ethanol, filter, spray-dry the filtrate, micronize the particle size to 3 μm, mix with 15 g of crystalline lactose with a particle size of 30 μm, and then use a 200-mesh sieve to mix 3 times and then sterilize. Pack into No. 2 capsules, each capsule contains 1 mg of triamcinolone acetonide acetate. Electron microscope observation shows that the particles are spherical.
[0047] The process conditions are as follows: inlet temperature is 105°C, outlet temperature is 70°C, air flow rate is 90%, nozzle outlet inner diameter is 0.1cm, nozzle air flow rate is 800ml / min, and sample injection speed is 50mL / h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com