Separation-type water suspension medicament for treating dermatopathy
A technology for skin diseases and drugs, applied in the field of skin drugs, can solve the problems of particle instability and inability to ensure long-term storage of particle suspensions, and achieve the effects of reducing waste, ensuring suspension stability, and being easy to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] 1. Preparation of drug particles for transdermal absorption
[0029] The capsules packed with the pharmaceutical microparticles obtained in Examples 1 to 10 are plant capsules, and the capsules are packed in aluminum foil blisters after packing.
Embodiment 1
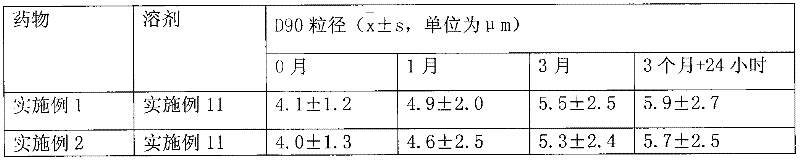
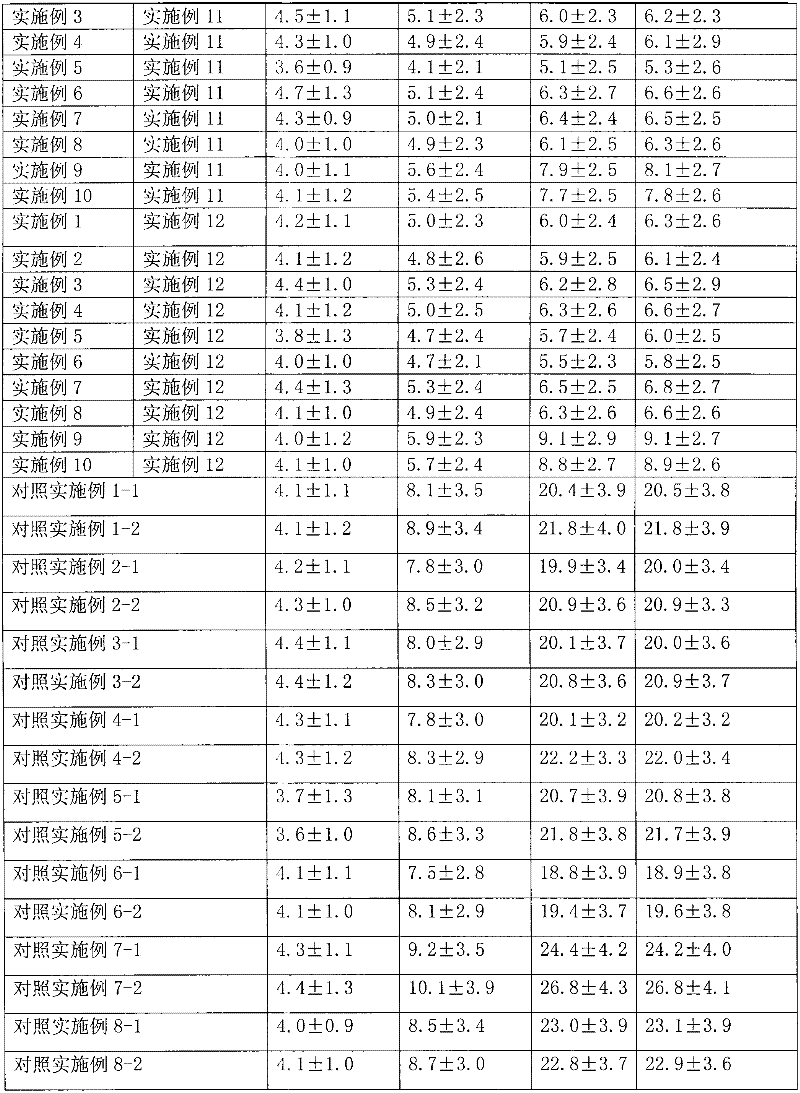
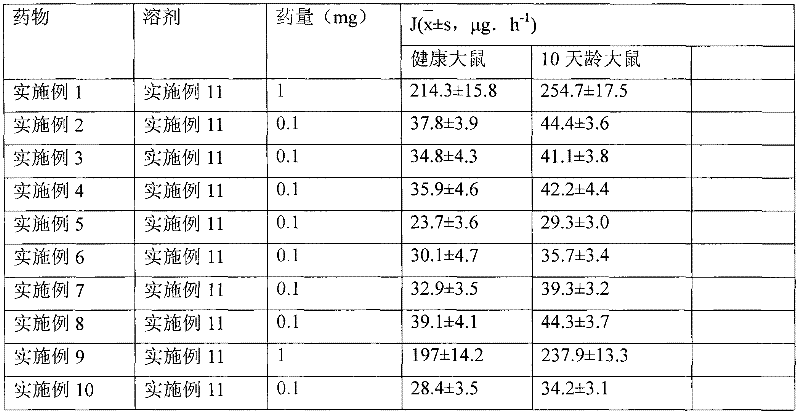
[0031] Dissolve 1g of hydrocortisone in ethanol, filter, spray-dry the filtrate, micronize the particle size to 3μm, pass through a 200-mesh sieve for 3 times, mix well, sterilize, and pack in No. 3 capsules, each capsule has hydrogenation Cortisone 10mg. Electron microscope observation shows that the particles are spherical.
[0032] The process conditions are: the inlet temperature is 105°C, the outlet temperature is 68°C, the air flow rate is 90%, the inner diameter of the nozzle outlet is 0.1cm, the air flow rate of the nozzle is 800ml / min, and the injection speed is 50mL / h
Embodiment 2
[0034] Dissolve 1 g of triamcinolone acetonide acetate in ethanol, filter, spray-dry the filtrate, micronize it to make the particle size reach 3 μm, mix it with a 200-mesh sieve for 3 times, sterilize it, and pack it into No. 3 capsules. Each capsule has Triamcinolone acetonide acetate 1 mg. Electron microscope observation shows that the particles are spherical.
[0035] The process conditions are as follows: inlet temperature is 105°C, outlet temperature is 70°C, air flow rate is 90%, nozzle outlet inner diameter is 0.1cm, nozzle air flow rate is 800ml / min, and sample injection speed is 50mL / h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com