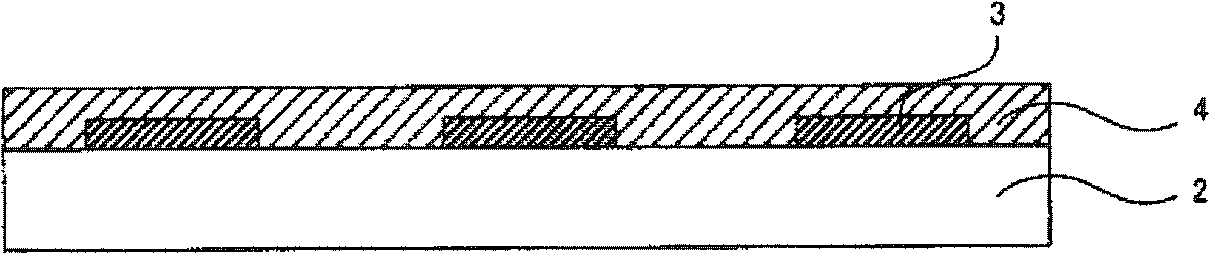
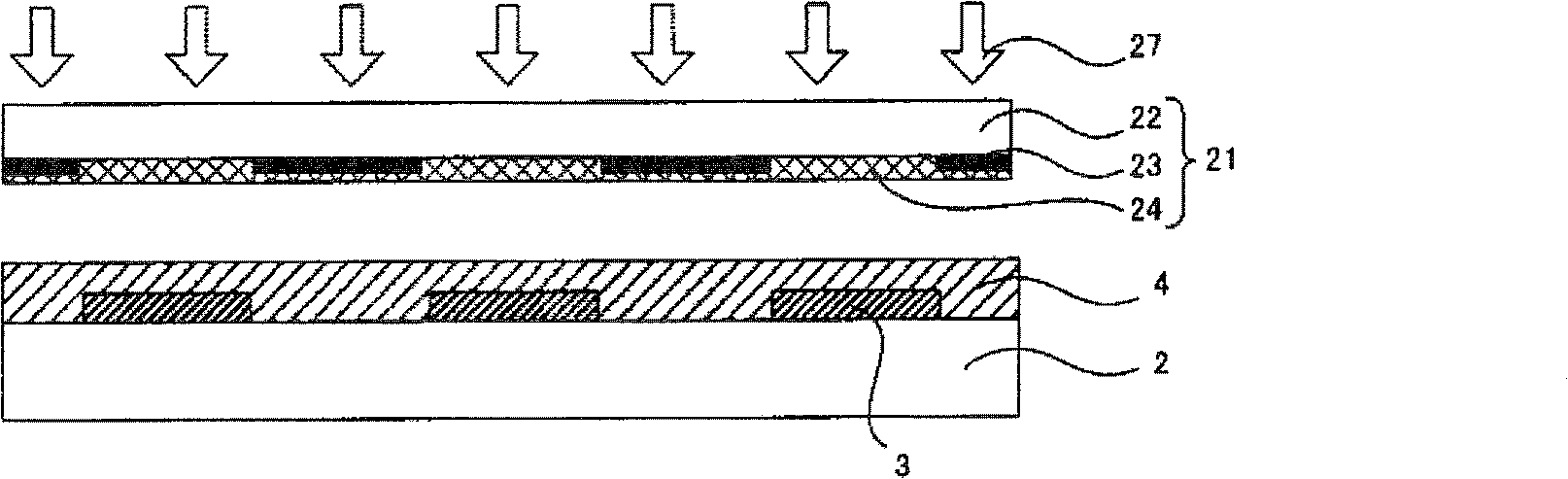
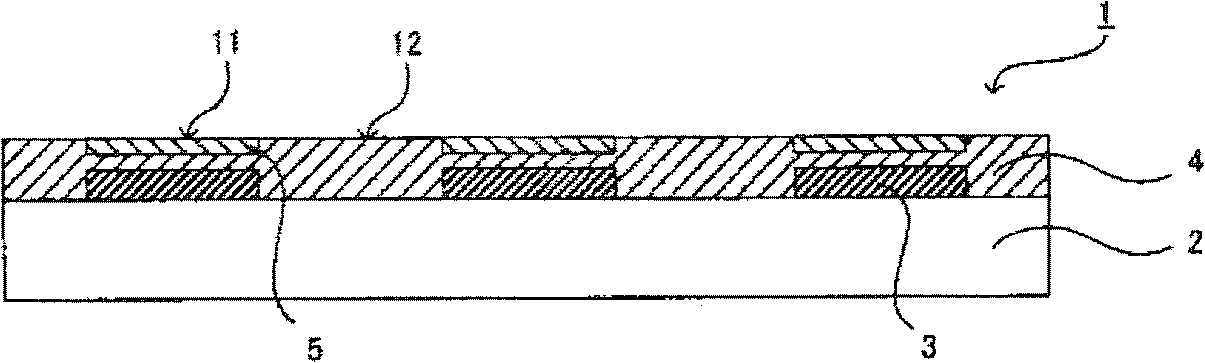
Device material for hole injection/transport layer, ink for forming hole injection/transport layer, device having hole injection/transport layer, and method for manufacturing same
A technology of hole injection and manufacturing method, which is applied in semiconductor/solid-state device manufacturing, electric solid-state devices, semiconductor devices, etc. It can solve problems such as material degradation and hole transport damage, and achieve high light resistance and life-span improvement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0383] Examples are given below to describe the present invention more specifically. These descriptions do not limit the present invention. In addition, in an Example, unless otherwise specified, a part means a weight part. In addition, the thickness of a layer or a film is represented by an average film thickness.
[0384] The present invention will be described more specifically by giving examples of organic EL elements below. It should be noted that the present invention is not limited to the description of the following examples.
[0385] The structures of the compounds obtained in the following synthesis examples were confirmed by 1H NMR (α-400 manufactured by JEOL Ltd.) and mass spectrometry (JMS600 manufactured by JEOL Ltd.). The NMR spectrum of the obtained compound is shown in Figures 13 to 16 .
Synthetic example 1
[0386] [Synthesis Example 1: Synthesis of Fluorine-Containing Organic Compound F-1]
[0387] 2, 2, 3, 3, 4, 4, 5, 5, 6, 6, 7, Fluorine-containing organic compound F-1 was synthesized by condensation reaction of 7,7-tridecafluoroheptanoic acid (manufactured by Aldrich) and 1,4-diaminobutane (manufactured by Tokyo Chemical Industry Co., Ltd.).
[0388] The molecular weight of the obtained compound F-1 was confirmed to be 434 by mass analysis, and it was confirmed by 1H NMR that a compound of the following structural formula was synthesized.
[0389] [chemical 5]
[0390]
Synthetic example 2
[0391] [Synthesis Example 2: Synthesis of Fluorine-Containing Organic Compound F-2]
[0392] In chloroform, in the presence of triethylamine, the condensation reaction of 1H, 1H, 2H, 2H-tridecafluoro-n-octyl iodide (manufactured by Tokyo Chemical Industry Co., Ltd.) and 2-aminoethanethiol (manufactured by Tokyo Chemical Industry Co., Ltd.), A fluorine-containing organic compound F-2 was synthesized. The molecular weight of the obtained compound F-2 was confirmed to be 423 by mass analysis, and it was confirmed by 1H NMR that a compound of the following structural formula was synthesized.
[0393] [chemical 6]
[0394]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com