Alkyl substituent-containing alicyclic dianhydride compound and polyimide prepared by same
A compound and alkyl technology, applied in the field of functional polyimide materials, can solve the problems of high cost and expensive PI alignment agent, and achieve the effect of improving heat resistance and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
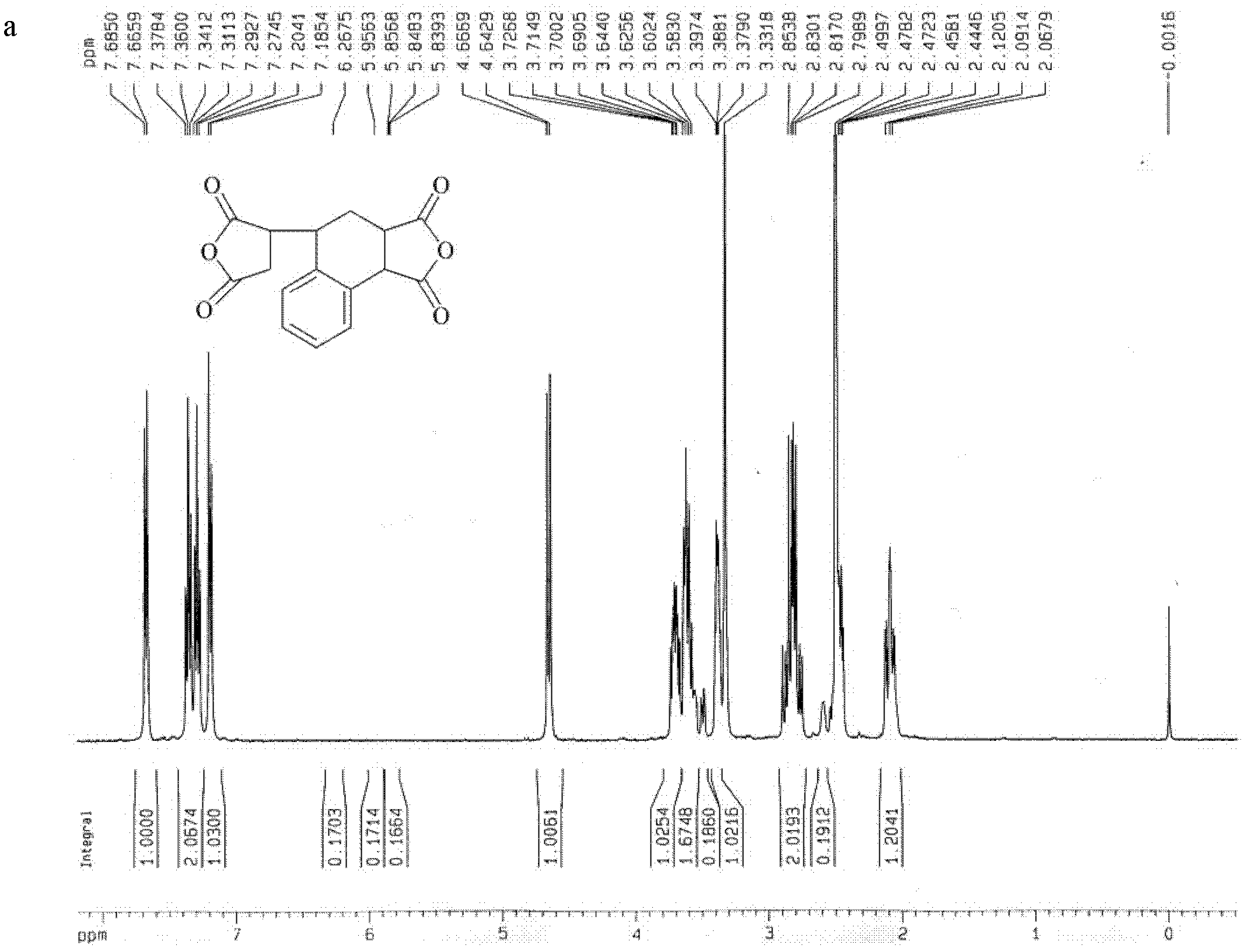
[0039] Embodiment 1, dianhydride compound 3, the synthesis of 4-dicarboxy-1,2,3,4-tetrahydro-1-naphthalene succinic dianhydride (TDA)
[0040] Add 250 g (2.55 mol) of maleic anhydride into a 250 mL three-necked flask equipped with a mechanical stirrer, a thermometer, and a gas inlet, and raise the temperature to blow nitrogen. After all of the solution was dissolved, 0.57 g (2.55 mmol) of a polymerization inhibitor 2,5-di-tert-butylhydroquinone was added. Add 398.1 g (3.82 mol) of styrene at one time. Change the nitrogen gas to nitrogen monoxide gas, raise the temperature to 115°C for reaction, and a precipitate appears after 1 hour. After reacting for 5 hours, cool down to 60°C, add 180ml of acetonitrile, reflux for 0.5h to dissolve the reaction system into a clear liquid, cool down slightly, add 200mL of toluene, and cool down. A large number of crystals were precipitated, filtered and washed three times with toluene to obtain loose white crystals with a yield of 90%.
[...
Embodiment 2
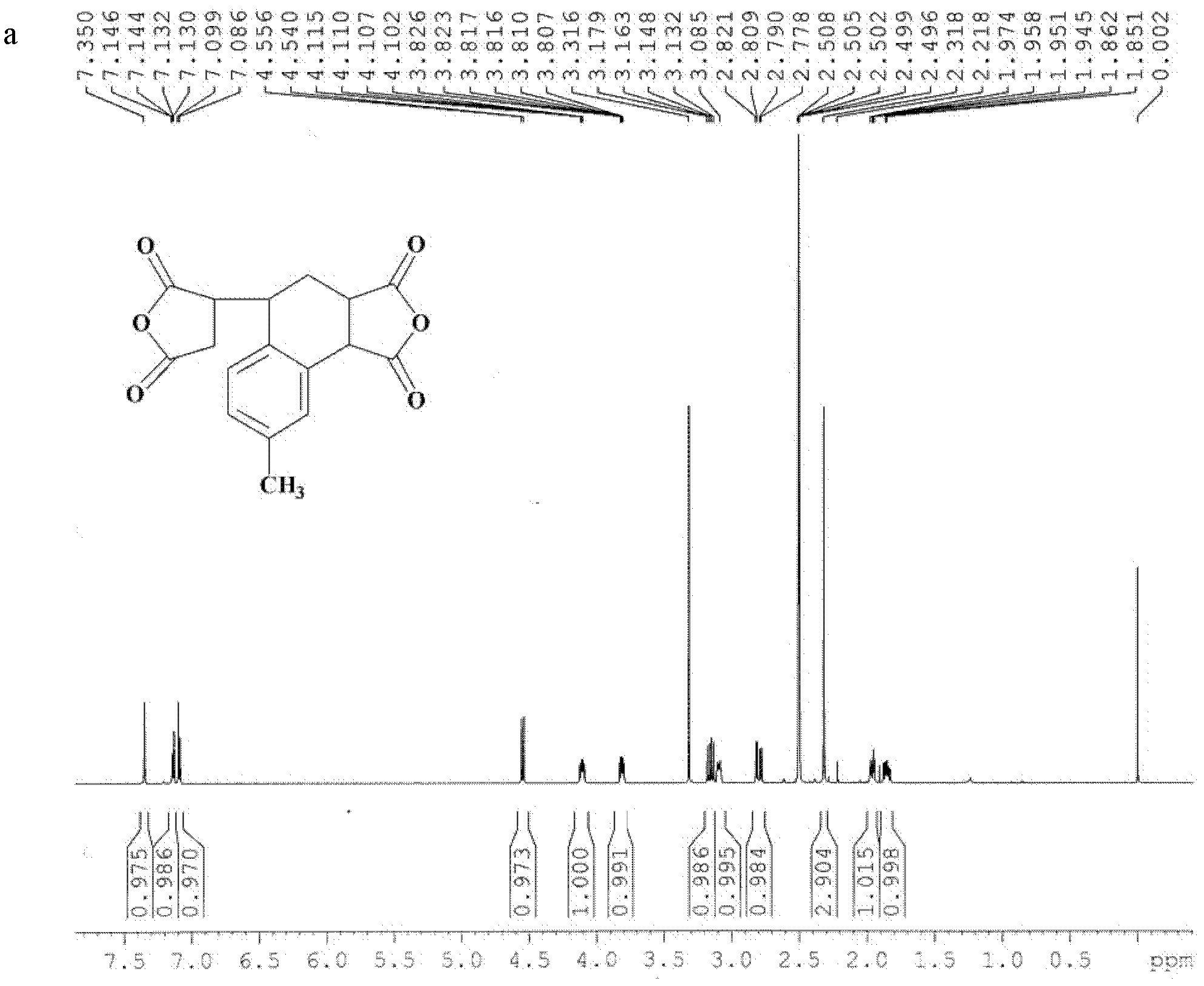
[0050] Embodiment 2, the synthesis of dianhydride compound 3,4-dicarboxy-1,2,3,4-tetrahydro-6-methyl-1-naphthalene succinic dianhydride (MTDA)
[0051] Add 43.75g (0.446mol) maleic anhydride, 80.60g (0.682mol) 4-methylstyrene, 0.1g (0.446mmol) ) 2,5-di-tert-butylhydroquinone, the temperature was raised to 120° C. under the catalysis of nitric oxide gas, and the reaction was maintained at constant temperature for 6 hours, and a large amount of orange solid was precipitated. Add 60 mL of acetonitrile, reflux for 0.5 h to dissolve the reaction system into a clear liquid, add 60 mL of toluene, and cool. A large number of crystals were precipitated, filtered, washed with toluene three times, and turned from orange to white. After washing with petroleum ether three times, loose white crystals were obtained with a yield of 89%.
[0052] The structure of this compound is shown in formula I, wherein R 1 =-H;R 2 =-CH 3 (m=1):
[0053]
[0054] The detection data are as follows ...
Embodiment 3
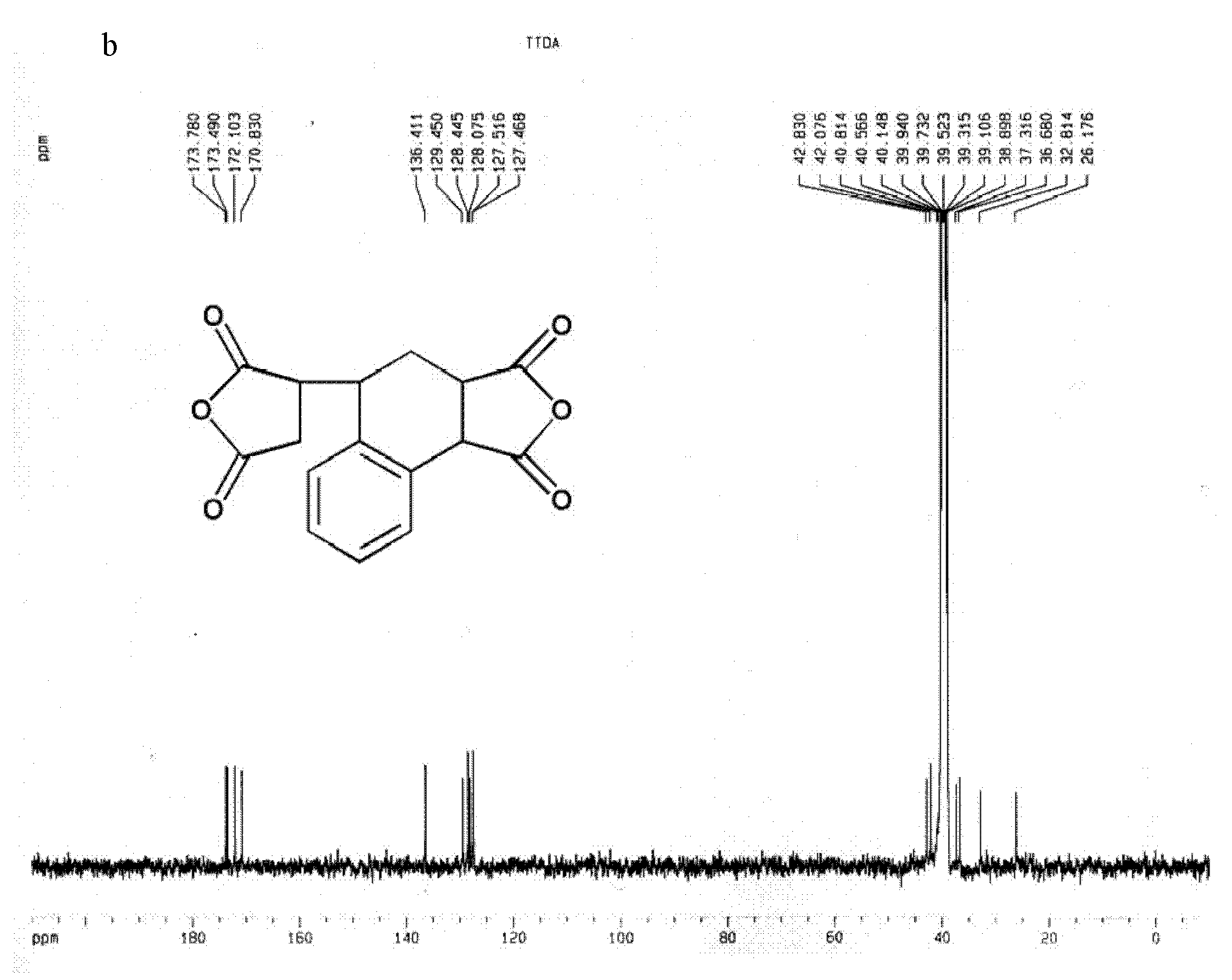
[0061] Embodiment 3, the synthesis of dianhydride compound 3,4-dicarboxy-1,2,3,4-tetrahydro-6-tert-butyl-1-naphthalene succinic dianhydride (TTDA)
[0062] Add 64.13g (0.654mol) maleic anhydride, 160.26g (1mol) 4-tert-butylstyrene, 0.22g (1mmol) 2 , 5-Di-tert-butyl hydroquinone, the temperature was raised to 120°C under the catalysis of nitric oxide gas, and the reaction was maintained at a constant temperature for 5 hours, and the system changed from a liquid phase to a solid phase. Add 250mL of acetonitrile, reflux until completely dissolved, and drop to room temperature. Add 200 mL of toluene, stand still to crystallize, and obtain a flocculent precipitate. After filtering and washing with toluene three times, the solid powder changed from orange to white. After washing with petroleum ether three times, a white powdery solid was obtained with a yield of 89%.
[0063] The structure of this compound is shown in formula I, wherein R 1 =-H;R 2 =-C 4 h 9 (m=4):
[0064] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com