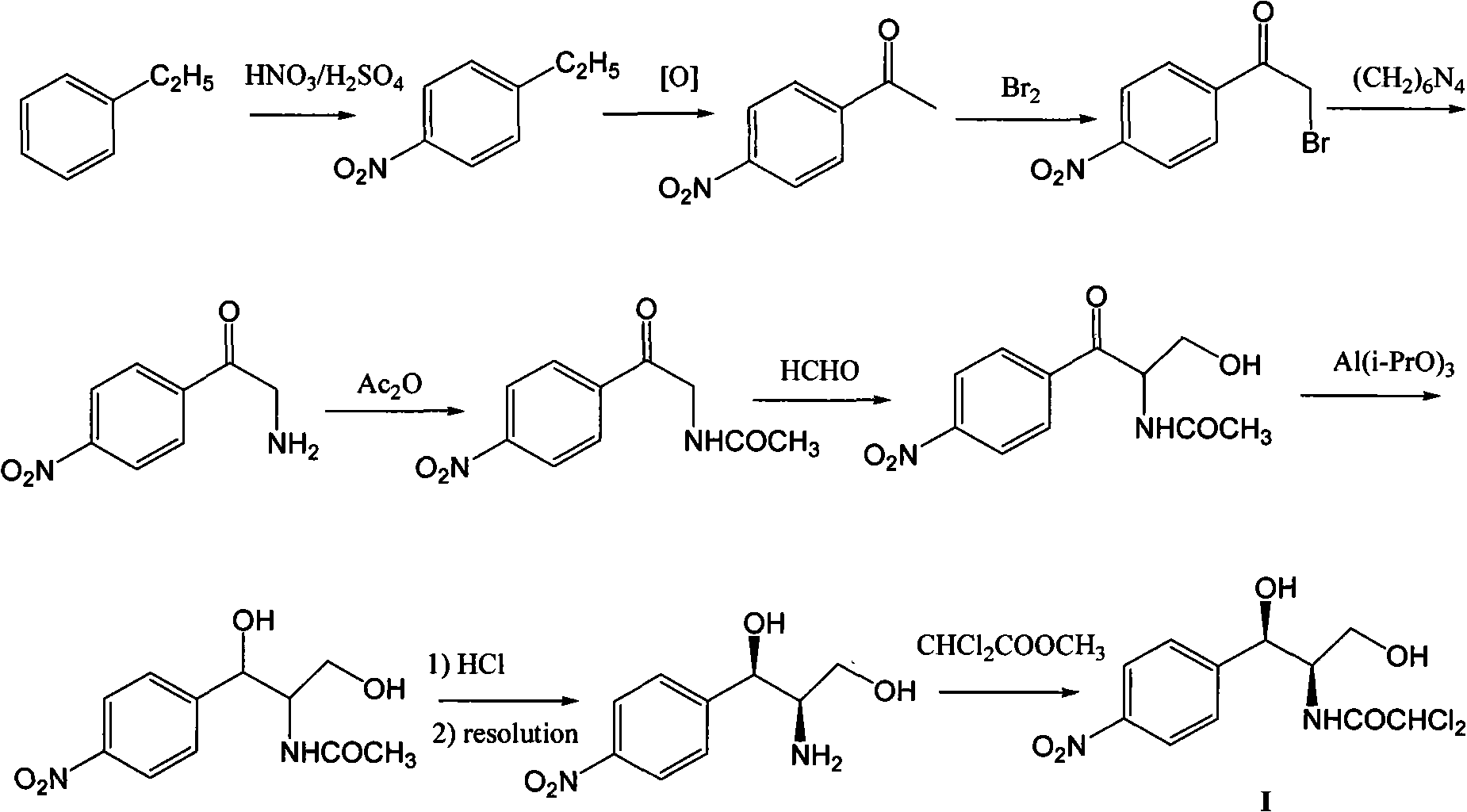
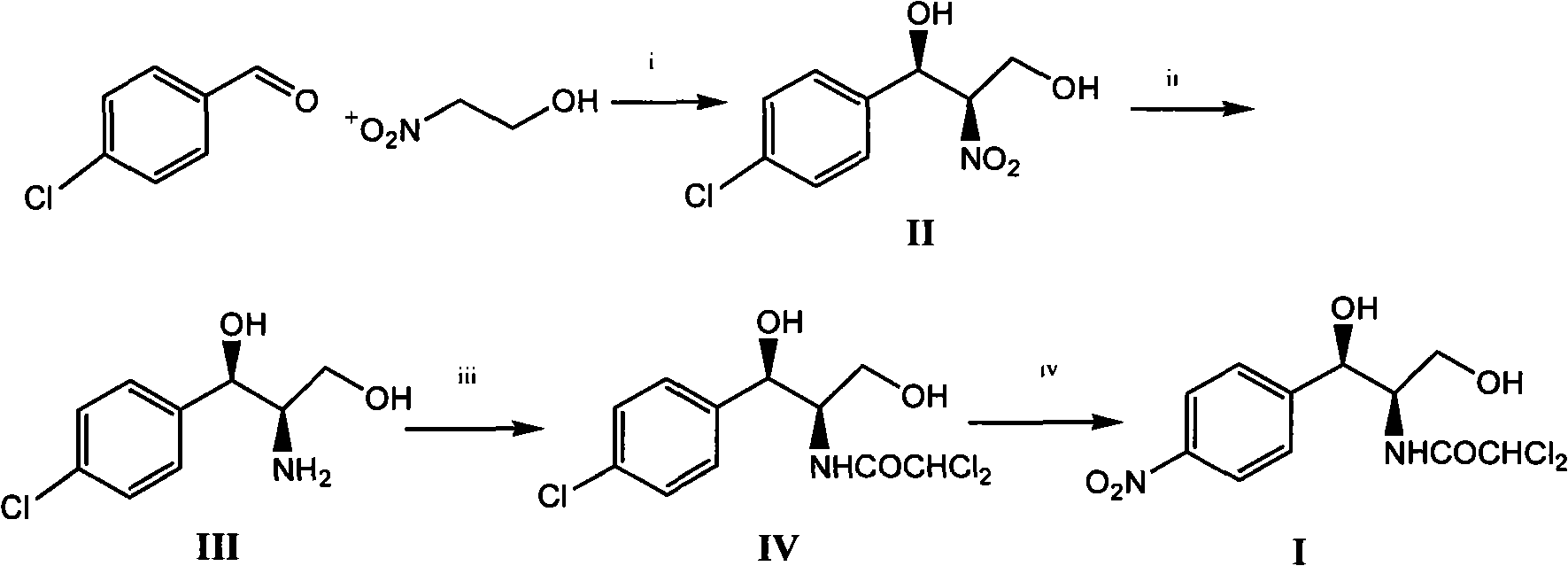
Method for synthesizing chloramphenicol from 4-chloro-benzaldehyde
A synthesis method and technology of chloramphenicol are applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, which can solve the problems of increased production cost and three wastes, long synthetic route of chloramphenicol, etc. The effect of easy availability of raw materials and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 (1R, 2R)-1 of the preparation of-2-nitro-1-(4-chlorophenyl)-1,3-propanediol
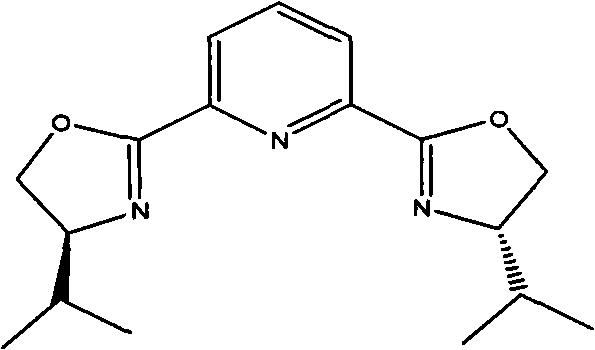
[0034] 1.8 g of Cu(OTf) 2 (0.50mmol), 2.4 grams of 2,6-bis[(S)-4-isopropyl-1-phenyl-4,5-dihydro-1H-2-imidazolyl]pyridine (5.2mmol) and 40 ml Add 1,4-dioxane into a 250 ml single-necked flask, replace the air inside with nitrogen and keep the nitrogen flow constant. At room temperature, stir magnetically for 2 hours and cool with an ice bath, then add 7.0 g of 4-chlorobenzaldehyde in sequence (50mmol), 45.5 grams of 2-nitroethanol (500mmol) and N-methylmorpholine (0.54 milliliters, 5.0mmol), the reaction solution was stirred in an ice bath at 0~5°C for about 24 hours, and no raw material was detected by thin-plate chromatography After 4-chlorobenzaldehyde spots, then the volatile solvent was removed by distillation under reduced pressure, the catalyst was removed by silica gel filtration, and the filtrate was concentrated to obtain 10.6 grams of product, the yield was 91.8%, and t...
Embodiment 2
[0035] Example 2 Preparation of (1R, 2R)-2-nitro-1-(4-chlorophenyl)-1,3-propanediol 2
[0036] 0.8 g of Cu(OTf) 2 (0.50mmol), 0.8 gram of rhodium acetate, 1.2 gram of 2,6-bis[(S)-4-isopropyl-1-phenyl-4,5-dihydro-1H-2-imidazolyl]pyridine (5.2 mmol) and 20 milliliters of 1,4-dioxane were added in a 250 milliliter single-necked flask, and the nitrogen flow was kept constant after replacing the air inside with nitrogen. At room temperature, magnetically stirred for 2 hours and then cooled with an ice bath, and then added 7.0 grams of 4-Chlorobenzaldehyde (50mmol), 45.5 grams of 2-nitroethanol (500mmol) and 0.15 grams of picoline, the reaction solution was stirred in an ice bath at 4°C for about 20 hours, and no raw material 4-chlorobenzaldehyde was detected by thin-plate chromatography After spotting, the volatile solvent was then distilled off under reduced pressure, the catalyst was removed by silica gel filtration, and the filtrate was concentrated to obtain 10.9 grams of prod...
Embodiment 3
[0037] Example 3 Preparation of (1R, 2R)-2-nitro-1-(4-chlorophenyl)-1,3-propanediol 3
[0038] 1.8 g of Cu(OTf) 2(0.50mmol), 2.4 grams of 2,6-bis[(S)-4-isopropyl-1-phenyl-4,5-dihydro-1H-2-imidazolyl]pyridine (5.2mmol) and 40 ml Add 1,4-dioxane into a 250 ml single-necked flask, replace the air inside with nitrogen and keep the nitrogen flow constant. At room temperature, stir magnetically for 2 hours and cool in an ice bath, then add 6.0 g of 4-bromobenzaldehyde in turn , 45.5 grams of 2-nitroethanol (500mmol) and N-methylmorpholine (0.54 milliliters, 5.0mmol), the reaction solution was stirred in an ice bath at 4°C for about 30 hours, and no raw material 4-chlorobenzaldehyde was detected by thin plate chromatography After spotting, the volatile solvent was then distilled off under reduced pressure, the catalyst was removed by silica gel filtration, and the filtrate was concentrated to obtain 9.6 grams of product, with a yield of 86.2%. The e.e value of HPLC was 92.9%. 1H), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com