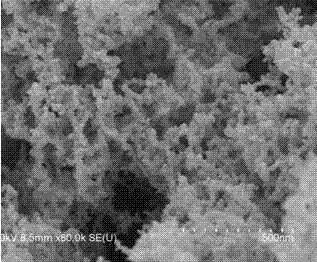
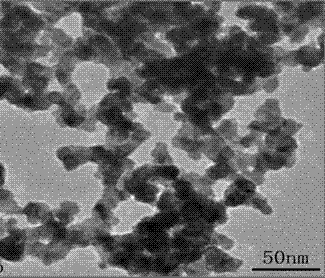
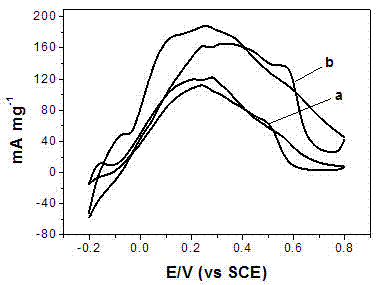
Method for preparing Pd catalyst with three-dimensional nano meshy structure by reduction of nitrile rubber precursor
A three-dimensional nano, network structure technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. , Pd metal catalyst instability and other problems, to achieve the effect of simple preparation method, high stability, excellent electrocatalytic activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The method for preparing the Pd catalyst with a three-dimensional nano-network structure by reducing the precursor of cyanide rubber comprises the following steps:
[0027] 1) Pipette 2.0 ml 0.05 mol / L K 2 PdCl 4 solution and 1.0 ml 0.05 mol / L K 3 Co(CN) 6 solution, ultrasonically mixed, and left to stand for half an hour to obtain a cyano rubber precursor.
[0028] 2) At room temperature (25°C), slowly add excess reducing agent solution (NaBH 4 ), let it stand for 24 hours, so that the cyano rubber precursor and the reducing agent react completely.
[0029] 3) After HClO 4 After washing with water several times, check with silver nitrate solution until there is no chloride ion in the eluate. Pd-NNWs were obtained after drying in a vacuum desiccator.
Embodiment 2
[0031] The method for preparing the Pd catalyst with a three-dimensional nano-network structure by reducing the precursor of cyanide rubber comprises the following steps:
[0032] 1) Pipette 2.0 ml 0.05 mol / L K 2 PdCl 4 solution and 1.0 ml 0.05 mol / L K 3 Co(CN) 6 solution, ultrasonically mixed, and left to stand for half an hour to obtain a cyano rubber precursor.
[0033] 2) At room temperature (25°C), slowly add excess reducing agent solution (NaH 2 PO 2 ), let it stand for 24 hours, so that the cyano rubber precursor and the reducing agent react completely.
[0034] 3) After HClO 4 After washing with water several times, check with silver nitrate solution until there is no chloride ion in the eluate. Pd-NNWs were obtained after drying in a vacuum desiccator.
Embodiment 3
[0036] The method for preparing the Pd catalyst with a three-dimensional nano-network structure by reducing the precursor of cyanide rubber comprises the following steps:
[0037] 1) Pipette 2.0 ml 0.05 mol / L K 2 PdCl 4 solution and 1.0 ml 0.05 mol / L K 3 Co(CN) 6 solution, ultrasonically mixed, and left to stand for half an hour to obtain a cyano rubber precursor.
[0038] 2) At room temperature (25°C), slowly add excess reducing agent solution (HCOOH) and let it stand for 24 hours to completely react the cyano rubber precursor with the reducing agent.
[0039] 3) After HClO 4 After washing with water several times, check with silver nitrate solution until there is no chloride ion in the eluate. Pd-NNWs were obtained after drying in a vacuum desiccator.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com