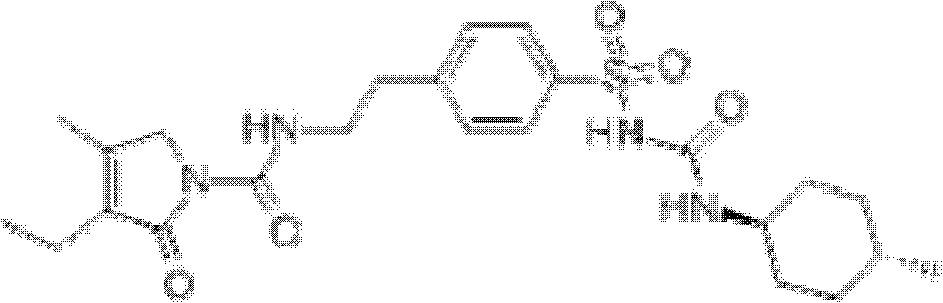Glimepiride dispersible tablet and preparation method thereof
A technology of glimepiride and dispersible tablets, applied in the field of medicine, can solve the problems of excessive particle size of raw materials, toxic and side effects, and high dosage of medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Glimepiride dispersible tablets (specification: 1mg / tablet; based on 10,000 tablets)
[0045]
[0046]
[0047] The preparation method adopts the traditional preparation method, and the steps are as follows:
[0048] (1) Micronizing glimepiride to prepare bulk drugs with different particle size ranges for use;
[0049] (2) Take the prescription amount of Povidone K 30 Put in a beating bucket, add 40% ethanol to make 1% povidone K 30 Ethanol solution, stir to dissolve completely, set aside;
[0050] (3) The prepared raw materials are mixed with the prescription amount of lactose, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, and sodium starch glycolate in an equal volume addition method. After mixing uniformly, add adhesive to make soft material. The prepared soft material has suitable humidity and viscosity, and it is advisable to "knead it into a dough and disperse when touched";
[0051] (4) Granulate the soft material with a 12-mesh screen a...
Embodiment 2
[0060] Example 2: Glimepiride dispersible tablets (specification: 2mg / tablet; based on 10,000 tablets)
[0061]
[0062]
[0063] The internal addition and external addition of the above-mentioned components are based on the amount of internal and external auxiliary materials used in the "internal and external addition of auxiliary materials" method of the present invention. The preparation method is as follows:
[0064] (1) Micronizing glimepiride to prepare APIs with different particle size ranges for use;
[0065] (2) Take the prescription amount of Povidone K 29 / 32 Put it in a beating bucket and add purified water to make 10% povidone K 29 / 32 Aqueous solution, stir to dissolve completely, set aside;
[0066] (3) The spare raw materials are mixed with the prescribed amount of internal adjuvants lactose, sodium starch glycolate, and povidone in an equal incremental manner. After mixing uniformly, add adhesive to make soft material. The prepared soft material has suitable humidity a...
Embodiment 3
[0078] Example 3: Glimepiride dispersible tablets (specification: 1mg / tablet; based on 10,000 tablets)
[0079]
[0080] The preparation method is as follows:
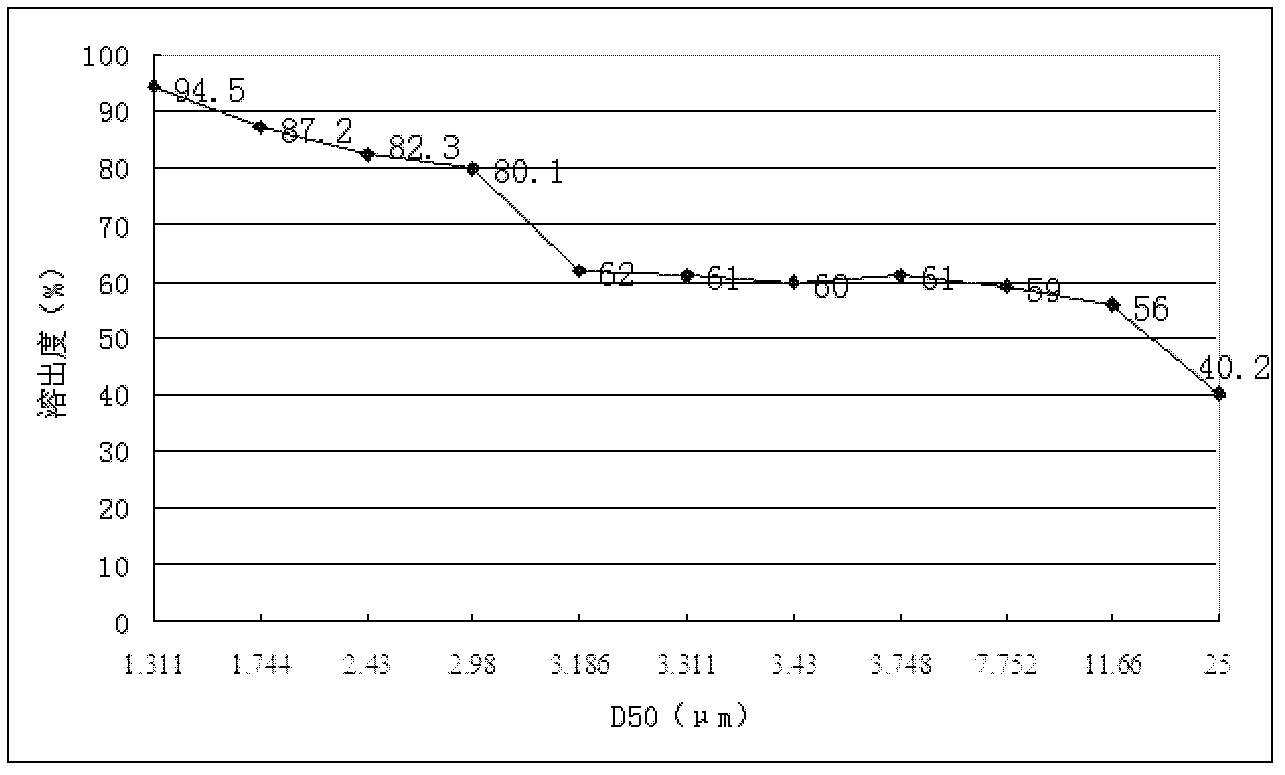
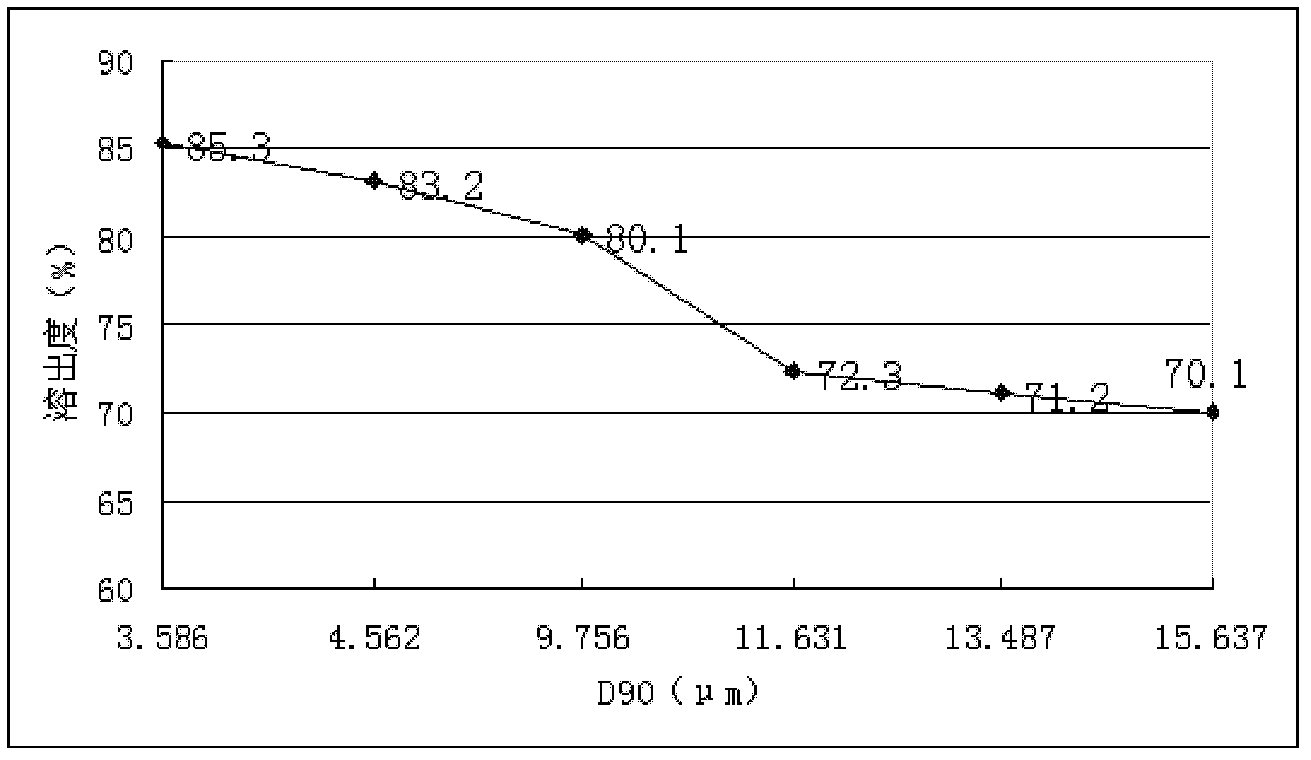
[0081] (1) Micronize glimepiride to make D 90 Less than 3.6μm, D 50 Between 1.5μm~1μm, spare;
[0082] (2) Take the prescription amount of Povidone K 30 Put in a beating bucket, add 40% ethanol to make 1% povidone K 30 Ethanol solution, stir to dissolve completely, set aside;
[0083] (3) The prepared raw materials are mixed with the prescription amount of lactose, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, and croscarmellose sodium in an equal volume addition manner. After mixing uniformly, add adhesive to make soft material. The prepared soft material has suitable humidity and viscosity, and it is advisable to "knead it into a dough and disperse when touched";
[0084] (4) Granulate the soft material with a 12-mesh screen and dry at 40℃;
[0085] (5) Whole grain, the mesh number of the screen is 10 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| D90 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com