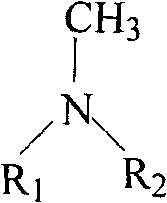
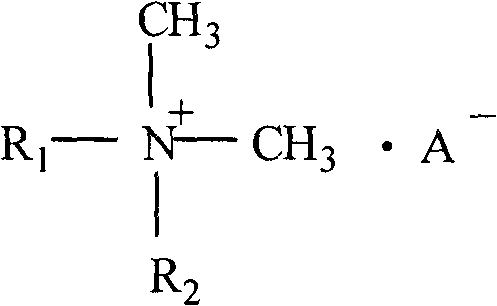
Dialkyl dimethyl quaternary ammonium salt and preparation method thereof
A technology of dialkyl dimethyl quaternary ammonium salt and dialkyl dimethyl ammonium carbonate is applied in the preparation of amino-substituted functional groups, the preparation of amino compounds from amines, organic chemistry, etc., which can solve the residual toxicity and is difficult to prepare other Acid anion quaternary ammonium salt, environmental pollution products and other problems, to achieve the effect of excellent surface activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Weigh 118g of trimethyl tertiary amine, 180.0g of dimethyl carbonate and 14.9g of methanol into the reaction kettle, slowly raise the temperature to 80°C, and stir for 3h. After the reaction, methanol and dimethyl carbonate were distilled off under reduced pressure to obtain tetramethyl ammonium methyl carbonate. Weigh 14.9g of tetramethylammonium methylammonium carbonate and 14.9g of water, add them into the flask, and stir and react at 30°C for 0.5h. After the reaction was finished, the tetramethylammonium bicarbonate product was obtained after the solvent was removed by distillation.
Embodiment 2
[0024] Weigh 143g of bis-butylmethyl tertiary amine, 180g of dimethyl carbonate and 32.3g of methanol, add them into the reaction kettle, slowly raise the temperature to 100°C, and stir for 5h. After the reaction, methanol and dimethyl carbonate were distilled off under reduced pressure to obtain dibutyl dimethyl ammonium methyl carbonate. Weigh 23.3g of dibutyldimethylammonium methyl ammonium carbonate, 7g of n-propanol and 10.1g of hydrochloric acid, add them into a flask, and stir at 30°C for 0.5h. After the reaction is finished, the dibutyl dimethyl ammonium chloride product is obtained after distilling off the solvent.
Embodiment 3
[0026] Weigh 127.5g bis-octaalkylmethyl tertiary amine, 225g dimethyl carbonate and 32.5g methanol, add them into the reaction kettle, slowly raise the temperature to 110°C, and stir for 6h. After the reaction was completed, methanol and dimethyl carbonate were distilled off under reduced pressure to obtain bis-octaalkyl dimethyl ammonium methyl carbonate. Weigh 34.5g of bis-octaalkyldimethylammonium methyl ammonium carbonate, 27.6g of absolute ethanol and 9.0g of sulfuric acid, add them into the flask, and stir and react at 40°C for 1.5h. After the reaction was finished, the dicarbon octaalkyl dimethyl ammonium bisulfate product was obtained after the solvent was removed by distillation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com