Pharmaceutical composition of prasugrel hydrobromide acetate compound
A technology of triamyl hydrobromide and acetic acid compounds, applied in the field of medicine, can solve the problems of unresponsiveness, lack of tolerance, and unsuitability for patients who are prone to bleeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
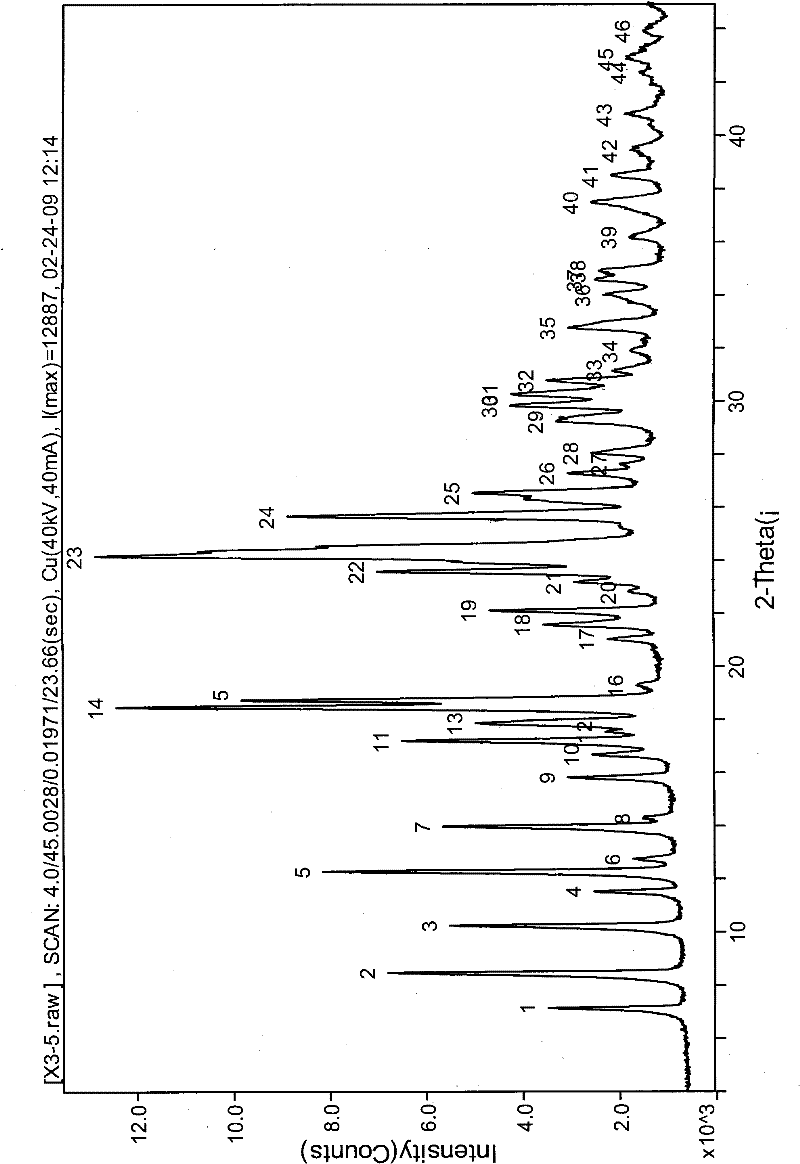
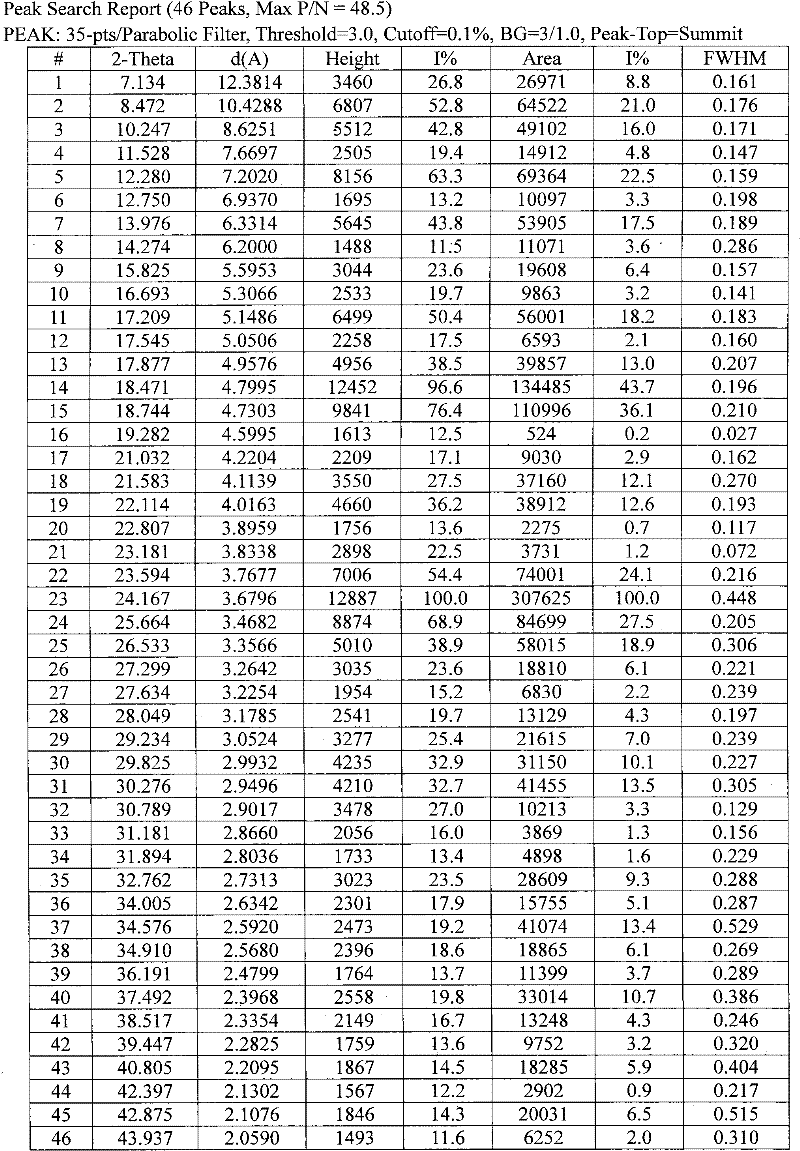
[0035] Example 1: Preparation and identification of prasugrel hydrobromide acetic acid solvated crystals
[0036] 2-Acetoxy-5-(α-cyclopropylcarbonyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine (2 g) was dissolved in acetic acid (20m1), add hydrogen bromide in acetic acid (40%) solution (1.1g) dropwise at room temperature 25°C under stirring, add a small amount of seed crystals, and stir at the same temperature for 2 hours. The precipitated crystals were filtered, washed with a small amount of acetone, and dried under reduced pressure at 60° C. for 4 hours to obtain white crystals. (product 2.29g, yield 83%)
[0037] Prasugrel hydrobromide acetic acid solvate (0.4 g) was dissolved in acetic acid (4 ml) and heated to 70°C. Stir for 10 min, then cool to room temperature naturally, and continue stirring for 2 hours. The precipitated crystals were filtered, washed with a small amount of acetone, and dried under reduced pressure at 60°C for 4 hours to obtain the title...
Embodiment 2
[0040] Embodiment 2: Solubility experiment
[0041] Source of tested samples:
[0042] Prasugrel hydrochloride crystals were prepared according to the method provided in Example 1 of CN1452624A;
[0043] Prasugrel hydrobromide is prepared according to the method provided in Example 1 of CN101255169A;
[0044] The crystal of prasugrel hydrobromide acetate was prepared according to the method of Example 1;
[0045] Experimental method: refer to the general examples in the second part of the Pharmacopoeia of the People's Republic of China in 2005
[0046] substance
Embodiment 3
[0048] prescription:
[0049]
[0050] 1) Pulverizing prasugrel hydrobromide acetate through a 100-mesh sieve for subsequent use;
[0051] 2) Pass microcrystalline cellulose and sodium carboxymethyl starch through a 80-mesh sieve, heat to control the water content to be no more than 6%, and set aside; pass stearic acid through a 100-mesh sieve for use;
[0052] 3) Mix the prescribed amount of prasugrel hydrobromide acetate with microcrystalline cellulose in an equal increment method, after increasing 3 times, then mix with the remaining amount of microcrystalline cellulose, sodium carboxymethyl starch and stearic acid ;
[0053] 4) Tablet pressing, that is, plain tablets;
[0054] 5) The stomach-soluble film coating premix is formulated into a coating liquid, stirred evenly, and set aside;
[0055] 6) Remove the fine powder from the plain tablet and perform film coating, inspection and packaging to obtain the coated tablet.
[0056] The water content of the sample was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com