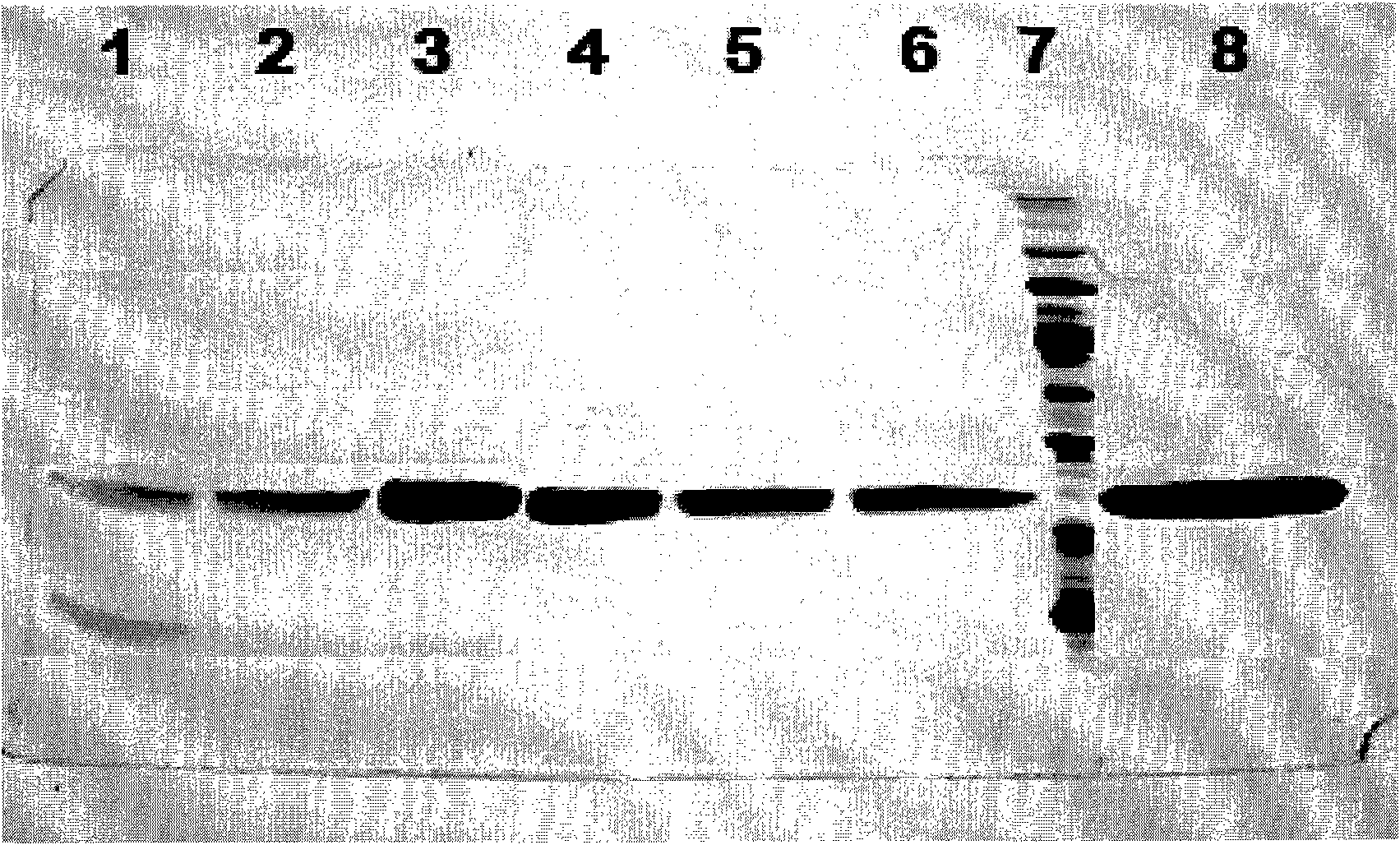Method for producing recombinant human bone morphogenetic protein-2 mature peptide
A morphogenetic protein and production method technology, applied in the field of protein purification, can solve problems such as the inability to achieve efficient industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037] Example 1 Solubilization of inclusion bodies
[0038] Dissolve the inclusion bodies in the lysate (7M guanidine hydrochloride, 50mM Tris-HCl, pH8.0, 1mM EDTA, 10mM DTT) at a ratio of 1 gram of inclusion body: 20mL of lysate, and stir at room temperature for more than 2 hours or at 4°C overnight. Then centrifuge at 12000rpm and 4°C for 20min, discard the precipitate and take the centrifugation supernatant. Protein concentration was determined by Bradford method.
[0039]Or solubilize by the following method. Dissolve inclusion bodies in lysate (8M urea, 50mM Tris-HCl, pH8.5, 1mM EDTA, 10mM DTT) at a ratio of 1 gram of inclusion body: 20mL of lysate, and stir at room temperature for more than 2 hours or overnight at 4°C . Then centrifuge at 12000rpm and 4°C for 20min, discard the precipitate and take the centrifugation supernatant. Protein concentration was determined by Bradford method.
example 2
[0040] Example 2 Renaturation of Inclusion Body
[0041] Refolding by dilution method. The refolding solution consists of: 100mM Tris-HCl, 0.5M L-Arg, 0.5M Urea, 1M NaCl, 2mM EDTA, pH8.5-9.5, 1mM oxidized glutathione, 5mM reduced glutathione. Slowly add the inclusion body enhancement solution into the refolding solution, control the final protein concentration to 0.05-0.3 mg / mL, and refold under a constant temperature environment of 4-20 degrees for 1-20 days.
example 3
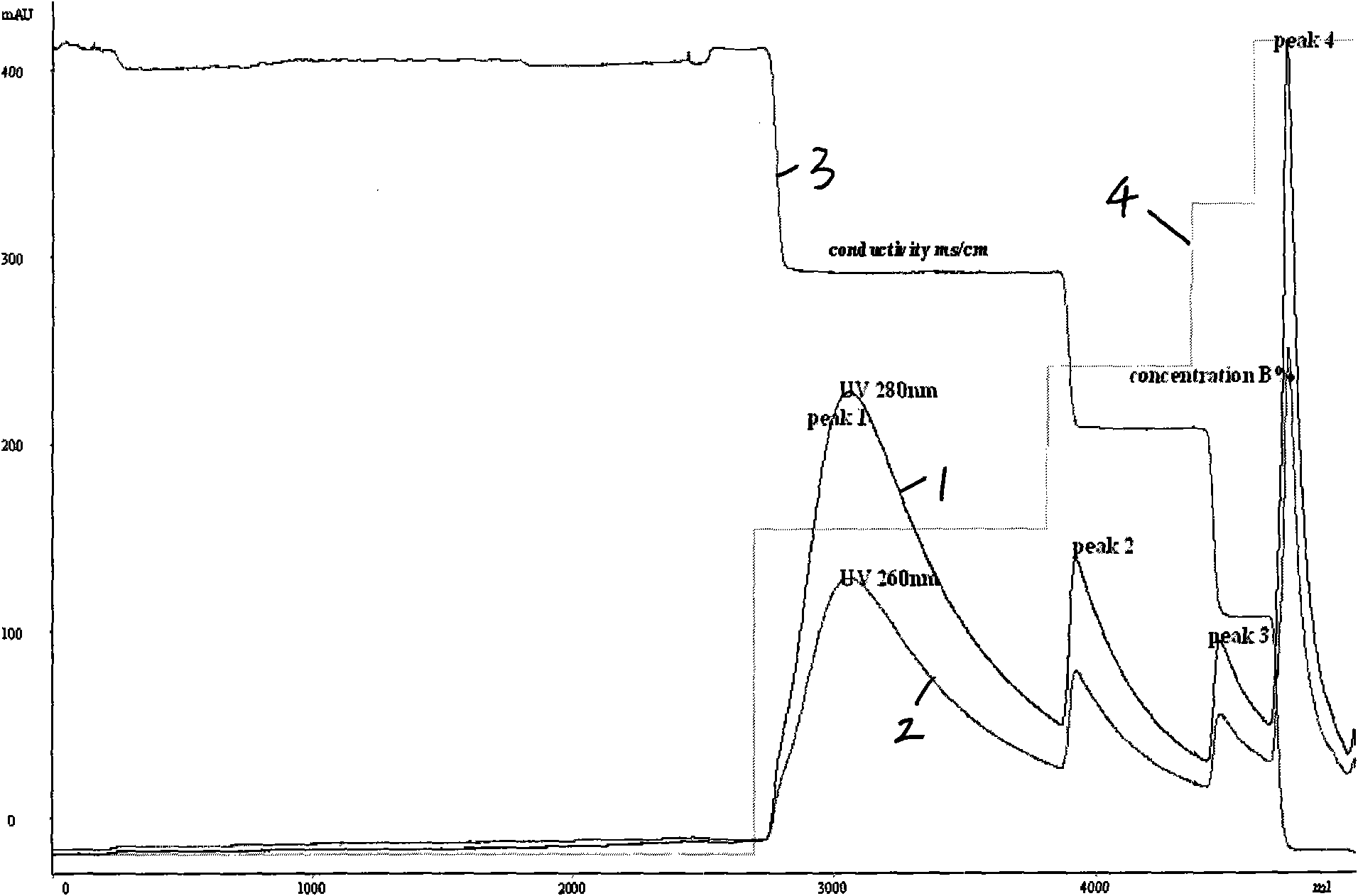
[0042] The hydrophobic interaction chromatography of example 3 refolding solution
[0043] Firstly, hydrophobic interaction chromatography with low salt concentration is used, the chromatography filler is phenylsepharose Fast Flow, and the equilibrium solution is phe-A: 0.5M urea, 25mM CHES, 15mM Tris, pH8.5, 5% N, N-dimethylformamide , 1M NaCl. The eluent was phe-B: 0.5Murea, 25mM CHES, 15mM Tris, pH 8.5, 5% N,N-dimethylformamide. First, use the balance solution phe-A to balance, and the refolding solution to directly load the sample. After the sample is loaded, continue to wash with the balance buffer phe-A until the UV reaches the baseline, and then use the elution buffer phe-B to gradually reduce the salt concentration. Elution was performed and the main peak was collected.
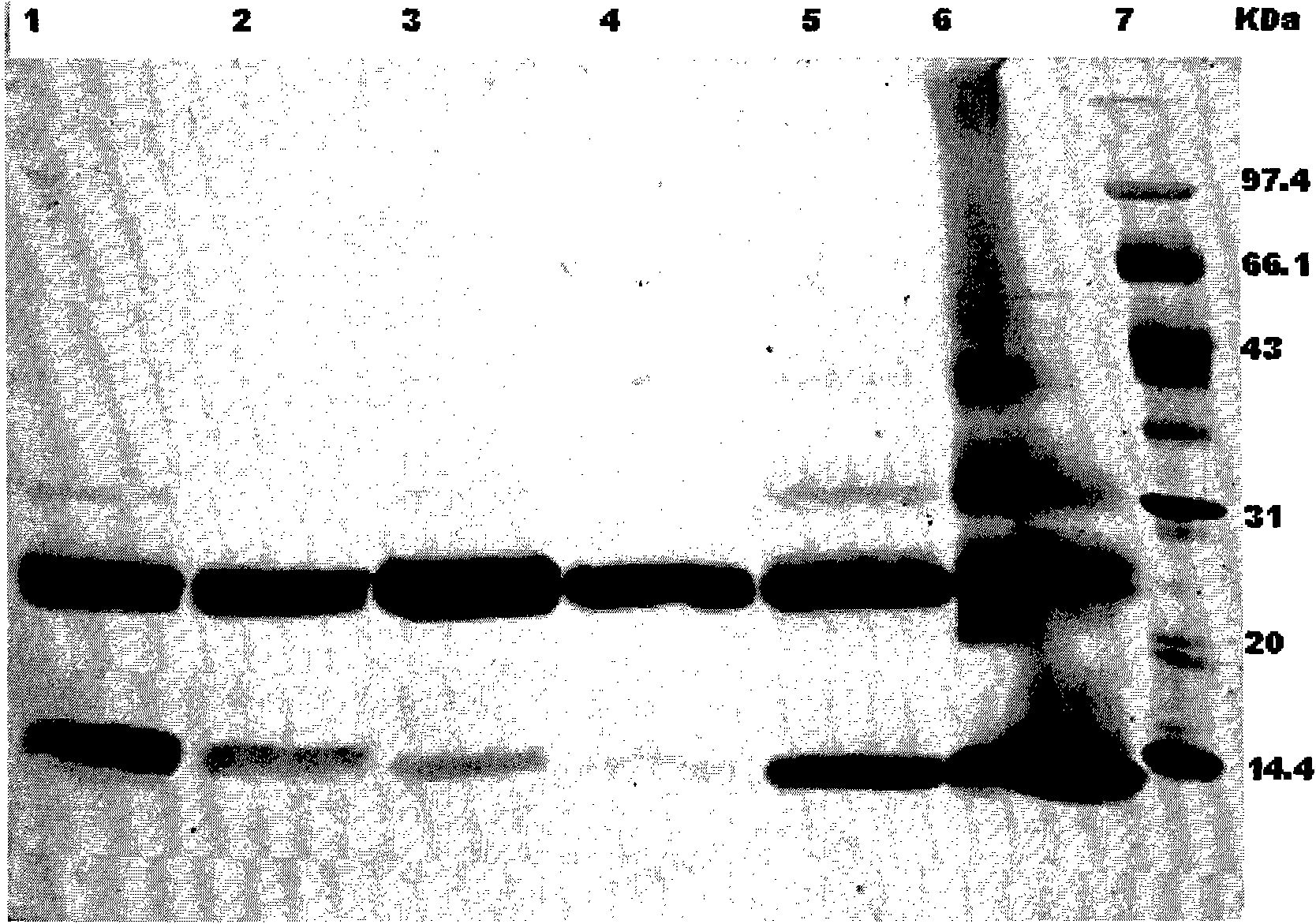
[0044] In order to further improve the purity, the hydrophobic interaction chromatography with high salt concentration can continue to be used for collecting the main peak. The chromatographic fill...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com