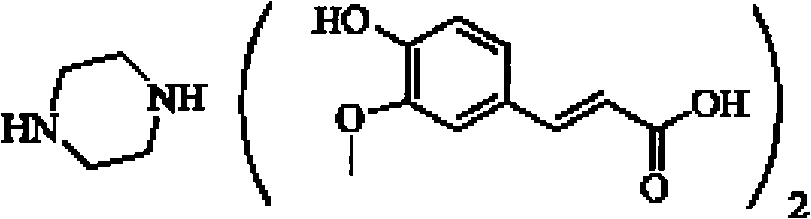
Piperazine ferulate sustained-release tablet and its preparation method
A technology of piperazine ferulic acid and sustained-release tablets, which is applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of not considering the release of the preparation and not mentioning the release results , the particle fluidity is not very good and other problems, to ensure the stability and durability, improve the bulk density and formability, maintain the effect of blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
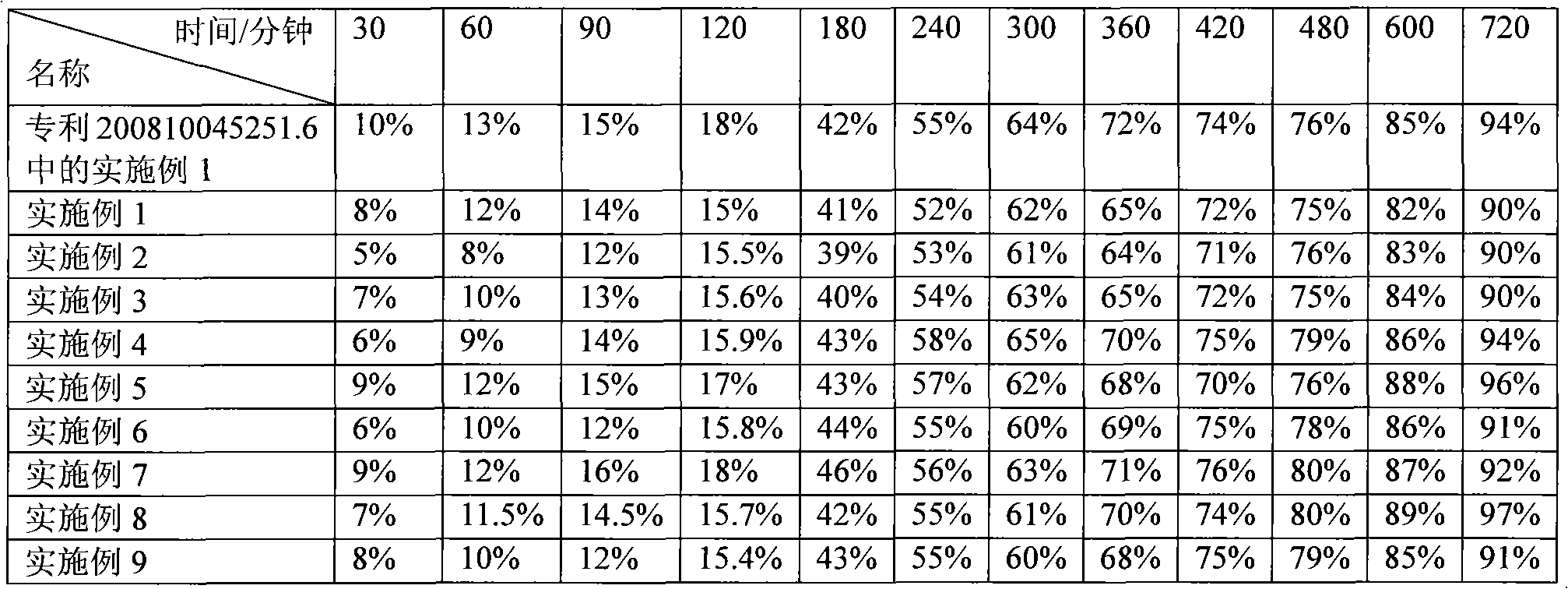
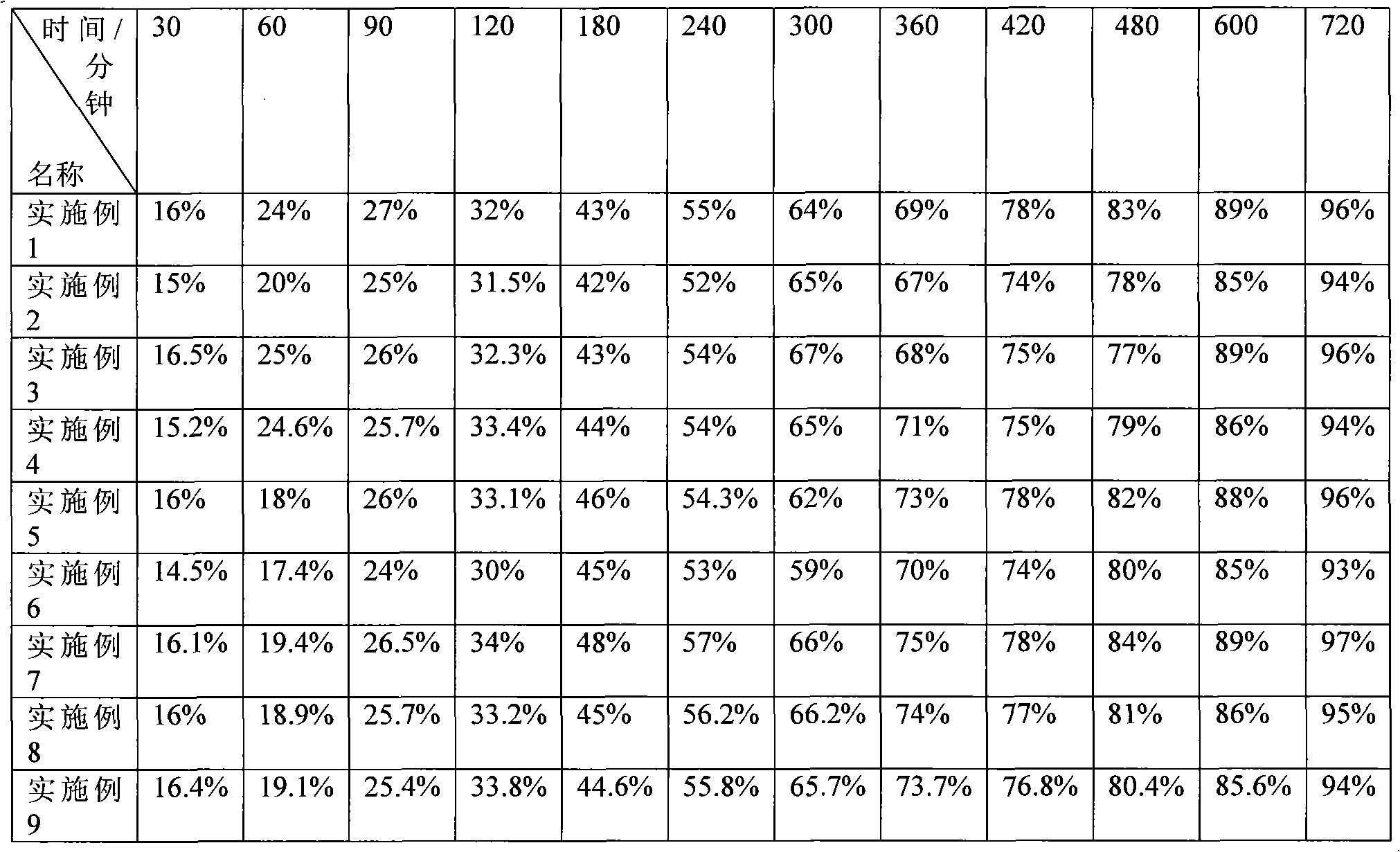
Examples
Embodiment 1
[0040] Example 1: Weigh 150 grams of piperazine ferulic acid, 60 grams of microcrystalline cellulose (PH101), 30 grams of Starch (Starch 1500, partially pregelatinized starch) and mix evenly, and use an appropriate amount of ethanol aqueous solution with a concentration of 80% or more As a binder, it is prepared into 24-30 mesh granules, dried at 50-55°C, granulated with a 24-30 mesh screen, and then 104 grams of hypromellose (K100LV), 3 grams of magnesium stearate, and micro-powdered silica gel are added. Mix 3g evenly, measure the bulk density, press into tablets, the tablet weight is 350mg / tablet, the hardness is kept at 50-100N, and the release rate is measured.
[0041] Bulk density: 0.44g / cm 3 ;Hardness: 63.5N.
Embodiment 2
[0042] Example 2: Weigh 150 grams of piperazine ferulic acid, 40 grams of microcrystalline cellulose (PH102), 20 grams of lactose, and 35 grams of Starch (Starch 1500, partially pregelatinized starch) and mix evenly, with a concentration of more than 80%. An appropriate amount of ethanol aqueous solution is used as a binder to prepare 24-30 mesh granules, dried at 50-55 ° C, and then added with 40 grams of hypromellose (K100LV) and hypromellose ( K15M) 10 grams, 2.5 grams of magnesium stearate, and 2.5 g of micropowdered silica gel were mixed uniformly, and the bulk density was measured, and pressed into tablets, with a tablet weight of 300 mg / tablet, and the hardness was maintained at 50 to 100 N to measure the release rate.
[0043] Bulk density: 0.45g / cm 3 ; Hardness: 77.0N.
Embodiment 3
[0044] Embodiment 3: Weigh 200 grams of piperazine ferulic acid, 60 grams of microcrystalline cellulose (PH102), 35 grams of Starch (Starch 1500, partially pregelatinized starch) and mix evenly, and use an appropriate amount of ethanol aqueous solution with a concentration of more than 80% as The adhesive is prepared into 24-30 mesh granules, dried at 50-55°C, granulated with a 24-30 mesh screen, and then 34 grams of hypromellose (K100LV) and 15 grams of hypromellose (K4M) are added , 4 grams of magnesium stearate, 3 grams of micro-powdered silica gel, mix evenly, measure the bulk density, press the tablet, the tablet weight is 350mg / tablet, the hardness is kept at 50-100N, and the release rate is measured.
[0045] Bulk density: 0.45g / cm 3 ; Hardness: 65.0N.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com