Polyethylene glycol-integrated interferon variant freeze-dried preparation
A technology of freeze-dried preparations and polyethylene glycol, which is applied in the direction of freeze-dried transportation, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., which can solve the problems of high price of human serum albumin, poor preparation stability, Problems such as poor resolubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of mPEG 20kDa -IFN-SA
[0039] IFN-SA solution (3.5mg / ml) is dissolved in sodium phosphate with a concentration of 100mM, pH6.0 contains 20mM NaCNBH 3 After cooling at 4°C, mix thoroughly, and add methoxypolyethylene glycol aldehyde (mPEG-propionaldehyde, average molecular weight 20kDa) in excess of 4 times the molar amount.
[0040] During the reaction, the modification degree of the protein was monitored by reversed-phase HPLC. After 10 hours, the reversed-phase HPLC analysis showed that 80% of the proteins with unblocked α-amino groups at the N-terminal were converted into mPEG-IFN-SA derivatives.
[0041] At the 10-hour time point, the reaction mixture was diluted 5-fold with water, and the purification of the mPEG-IFN-SA derivative was accomplished by ion-exchange chromatography on a Hiload 16 / 10 S Sepharose HP column buffered with 20 mM sodium acetate. The pH of the solution was balanced at 5.5, the reaction mixture was loaded into the colu...
Embodiment 2
[0042] Example 2 Configure the freeze-dried stock solution in the following proportions, and freeze-dry it into a finished product
[0043] Na 2 HPO 4 .12H 2 O, 2.58mg / ml, NaH 2 PO 4 .2H 2 O, 0.44mg / ml;
[0044] (1) 3.5% dextran (w / v) + 3.5% trehalose (w / v);
[0045] (2) 3.5% dextran (w / v) + 1.1% trehalose (w / v);
[0046] (3) 3.5% dextran (w / v) + 1.7% trehalose (w / v);
[0047] (4) 4% dextran (w / v) + 1% trehalose (w / v);
[0048] (5) 4% dextran (w / v) + 0.5% trehalose (w / v);
[0049] (6) 5.2% dextran.
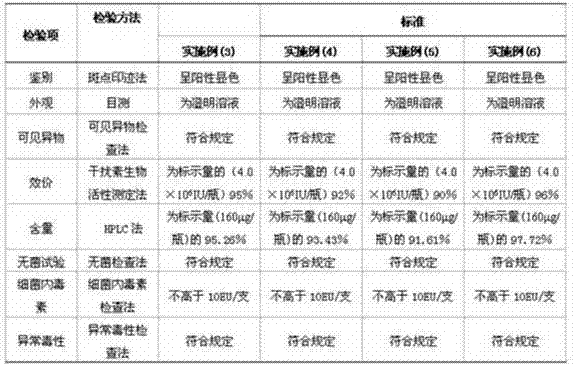
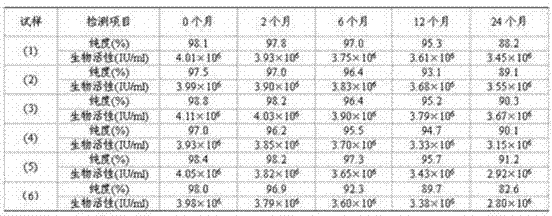
[0050] According to the proportion, take dextran or different proportions of dextran-trehalose, prepare it with phosphate buffer solution with a pH of 6.5-7.5, filter and sterilize, and prepare mPEG20 containing different proportions of dextran or dextran-trehalose cryoprotectant kDa - IFN-SA solution containing mPEG per ml of solution 20kDa -IFN-SA 50-160 μg / ml, mixed well, divided into 1ml / bottle, freeze-dried to prepare a freeze-dried preparation, and stored.
[00...
Embodiment 3
[0057] mPEG 20kDa -IFN-SA 160 μg / ml
[0058] Na 2 HPO 4 .12H 2 O 2.58mg / ml
[0059] NaH 2 PO 4 .2H 2 O 0.44mg / ml
[0060] Dextran 3.86mg / ml
[0061] Trehalose 1.24mg / ml
[0062] Preparation method and process:
[0063] Accurately prepare the diluent according to the following diluent formula, and use it to dilute the original solution after preparation. The utensils used must be treated without pyrogens, and the whole process is operated under sterile conditions. Diluent formula: dextran 3.86g, trehalose 1.24g, Na 2 HPO 4 12H 2 O 2.58 g, NaH 2 PO 4 2H 2 O 0.44 g, the volume for injection is determined to be 1 L, and the pH of the diluent is about 7.2
[0064] Take the qualified polyethylene glycol recombinant integrated interferon variant stock solution, accurately measure its volume, dilute with diluent to a protein concentration of 0.16 mg / ml, and filter aseptically to obtain a semi-finished product.
[0065] Sterile and pyrogen-fre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com