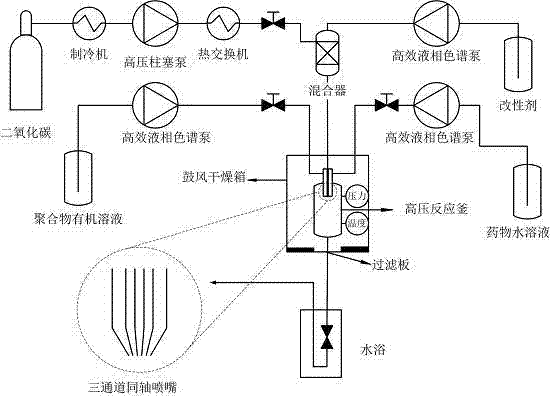
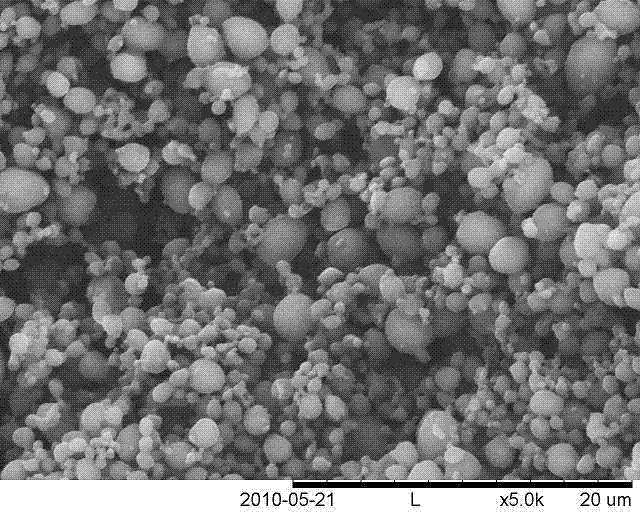
Method adopting supercritical CO2 fluid technology to produce water-soluble medicine controlled-release particles
A technology of water-soluble drugs and fluid technology, which is applied in the field of preparation of drug dosage forms, can solve the problems of inability to prepare water-soluble drug sustained-release particles, and achieve the effects of high drug activity retention, low toxicity, and no solvent residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0015] Example 1 Preparation of morphine-polylactic acid drug-loaded microspheres
[0016] (1) Take 4ml of Morphine Hydrochloride Injection 10mg / ml, dilute to 24ml with distilled water, and put it in a brown bottle for later use. Weigh 300 mg of poly-L-lactic acid with a molecular weight of 100 KDa, add 60 ml of organic solvent into the reaction flask, and stir to dissolve at room temperature. Prepare pure ethanol for analysis.
[0017] (2) Assemble the high-pressure reactor, turn on the supercritical fine particle device, start the refrigeration system, set the reaction temperature to 33°C, the pressure to 12MPa, CO 2 The pump flow rate is 300ml / min. Turn on the high-pressure plunger pump, constant temperature water bath box and blast drying oven to increase the pressure and temperature of the reaction kettle. Adjust the vent valve and the temperature control switch to keep the temperature and pressure of the reactor constant at the set value.
[0018] (3) Turn on the eth...
example 2
[0023] Example 2 Preparation of bovine serum albumin polylactic acid drug-loaded microspheres
[0024] (1) Weigh 20mg of bovine serum albumin (BSA) and dissolve it in 12ml of distilled water. Weigh 300mg of poly-L-lactic acid (PLLA) with a molecular weight of 10KDa, add 60ml of organic solvent into the reaction flask, and stir to dissolve at room temperature. Prepare pure ethanol for analysis.
[0025] (2) Assemble the high-pressure reactor, turn on the supercritical fine particle device, start the refrigeration system, set the reaction temperature to 33°C, the pressure to 12MPa, CO 2 The pump flow rate is 300ml / min. Turn on the high-pressure plunger pump, constant temperature water bath box and blast drying oven to increase the pressure and temperature of the reaction kettle. Adjust the vent valve and the temperature control switch to keep the temperature and pressure of the reactor constant at the set value.
[0026] (3) Turn on the ethanol pump, set the flow rate to 1ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com