Method for extracting whole peptidoglycan from bifidobacterium longum NQ-1501
A technology of Bifidobacterium longum and peptidoglycan, which is applied in pharmaceutical formulations, peptide/protein components, medical raw materials derived from bacteria, etc., can solve unfavorable large-scale process production and product quality control, large solvent usage, and production cycle The problem of lengthiness and other problems, to achieve suitable large-scale production, complete cell wall structure, good anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
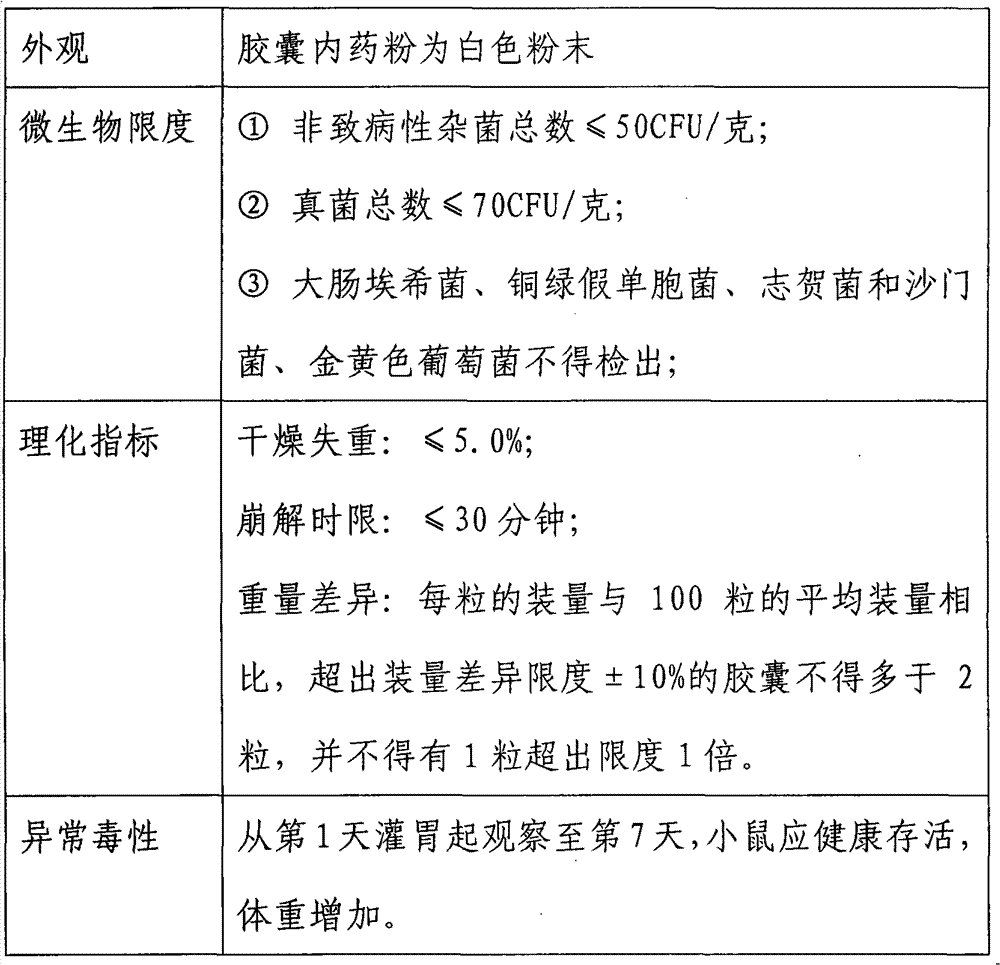
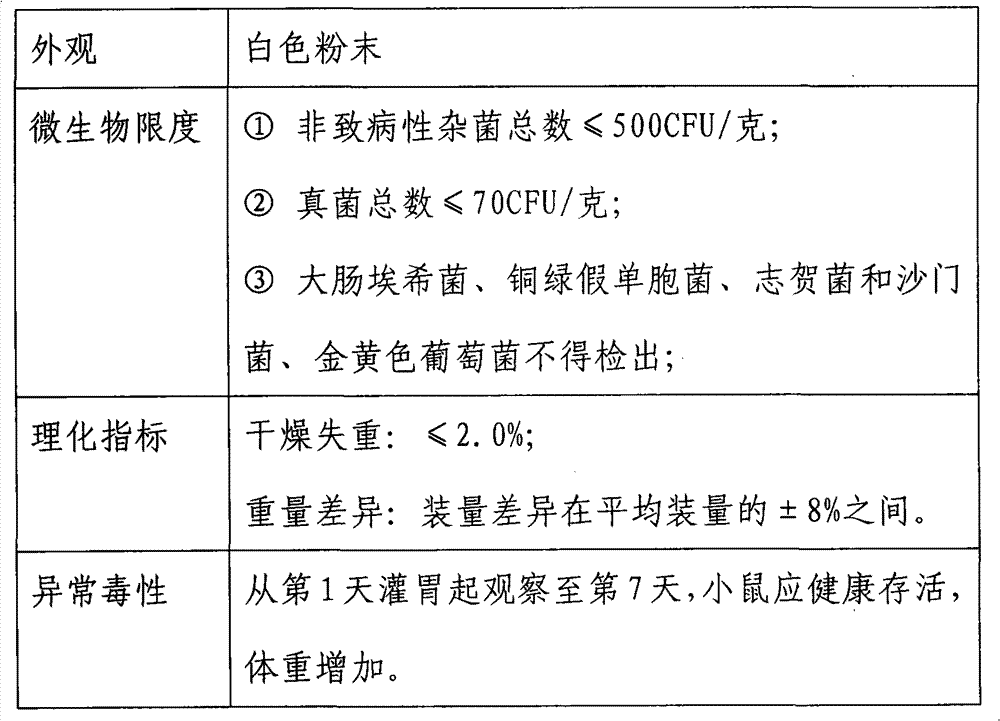
[0036] Remove the fatty substances on the cell membrane by degreasing, and then extract the teichoic acid of Bifidobacterium longum NQ-1501 strain with trichloroacetic acid to increase the permeability of the cell membrane, which is conducive to the better effect of external substances on the cell content. The protein substances in the cells are removed by enzymatic hydrolysis solution, and finally the complete peptidoglycan obtained by centrifugation to remove impurities and then freeze-dried can be seen through transmission electron microscopy. The structure of the cell wall skeleton is complete. Bifidobacterium longum NQ-1501 strain is available from public sales of Inner Mongolia Shuangqi Pharmaceutical Co., Ltd.
[0037] The preparation method of the complete peptidoglycan comprises the following steps:
[0038] (1) Washing of bacteria:
[0039] Take 100 grams of wet thallus of Bifidobacterium longum NQ-1501 strain, add 1000 milliliters of physiological saline and mix ev...
Embodiment 2
[0051] Structural integrity detection of a method for extracting complete peptidoglycan from Bifidobacterium longum NQ-1501 disclosed by the present invention
[0052] The original bacterium and the prepared complete peptidoglycan in Example 1 of the present invention were detected by electron microscopy. The results are shown in Figures 1 and 2. The complete peptidoglycan still retains the complete external structure of the original bacteria.
Embodiment 3
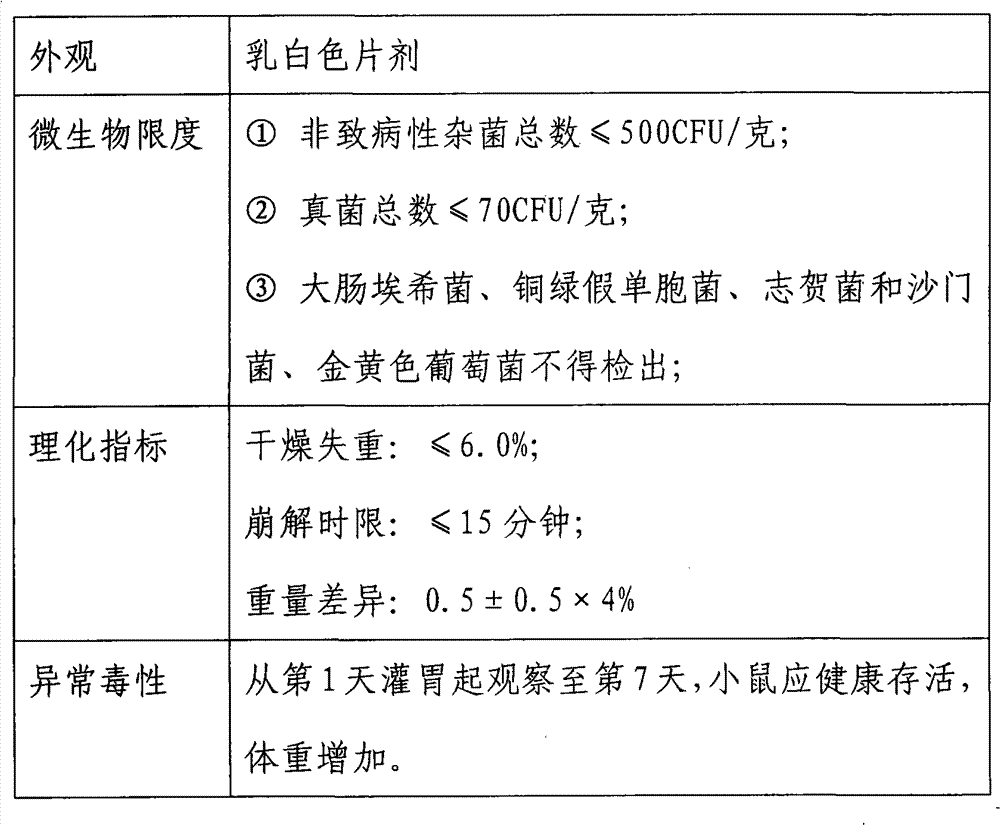
[0054] Application of tabletting technology, using glucose, lactose, microcrystalline cellulose, skim milk powder and other auxiliary materials as raw materials to prepare granules, and then mixing complete peptidoglycan powder (obtained in Example 1) and magnesium stearate for tabletting, and finally obtaining long double Fidobacterium NQ-1501 Complete Peptidoglycan Tablets.
[0055] Its production method includes the following steps:
[0056] (1) Preparation of pharmaceutical excipients: 0.125 g of glucose, 0.05 g of microcrystalline cellulose, 0.033 g of sodium starch glycolate, 0.0025 g of sucrose, 0.003 g of citric acid, 0.0625 g of skimmed milk powder, and 0.1875 g of lactose.
[0057] (2) Mixing of raw materials: 34.4 g of complete peptidoglycan was mixed with the above-mentioned auxiliary materials. Then add 0.0015 grams of magnesium stearate, after mixing, the raw material for direct compression can be obtained.
[0058] (3) Tablet compression: Put the mixed raw mat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com