Real-time reverse transcription-polymerase chain reaction (RT-PCR) detection method and kit for Coxsackie virus
A coxsackie virus and kit technology, applied in the field of molecular biology and nucleic acid detection, can solve the problem of limited group resolution, lack of serotype coxsackie virus high sensitivity, high specificity, flexible and simple detection method, poor sensitivity And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] 1 Materials and methods
[0118] 1.1 Materials and reagents
[0119] Inactivated coxsackievirus types A9, A16, B2, B3, and B5 and norovirus-containing GII stool samples were purchased from the Institute of Virology Prevention and Control, Chinese Center for Disease Control and Prevention, and serum types I, II, and III of poliovirus were inactivated. Live vaccine strain suspensions were obtained from the University of California, USA.
[0120] TIANamp Viral RNA Extraction Kit: Tiangen Biotechnology Co., Ltd., Cat.No.SD101; Prime Script TM Reverse transcription kit: Bao Biological Engineering Co., Ltd., Cat.No.DRR037A; Premix Ex Taq TM Real-time fluorescence amplification kit: Bao Biological Engineering Co., Ltd., Cat.No.DRR039A.
[0121] 1.2 Primers and probes
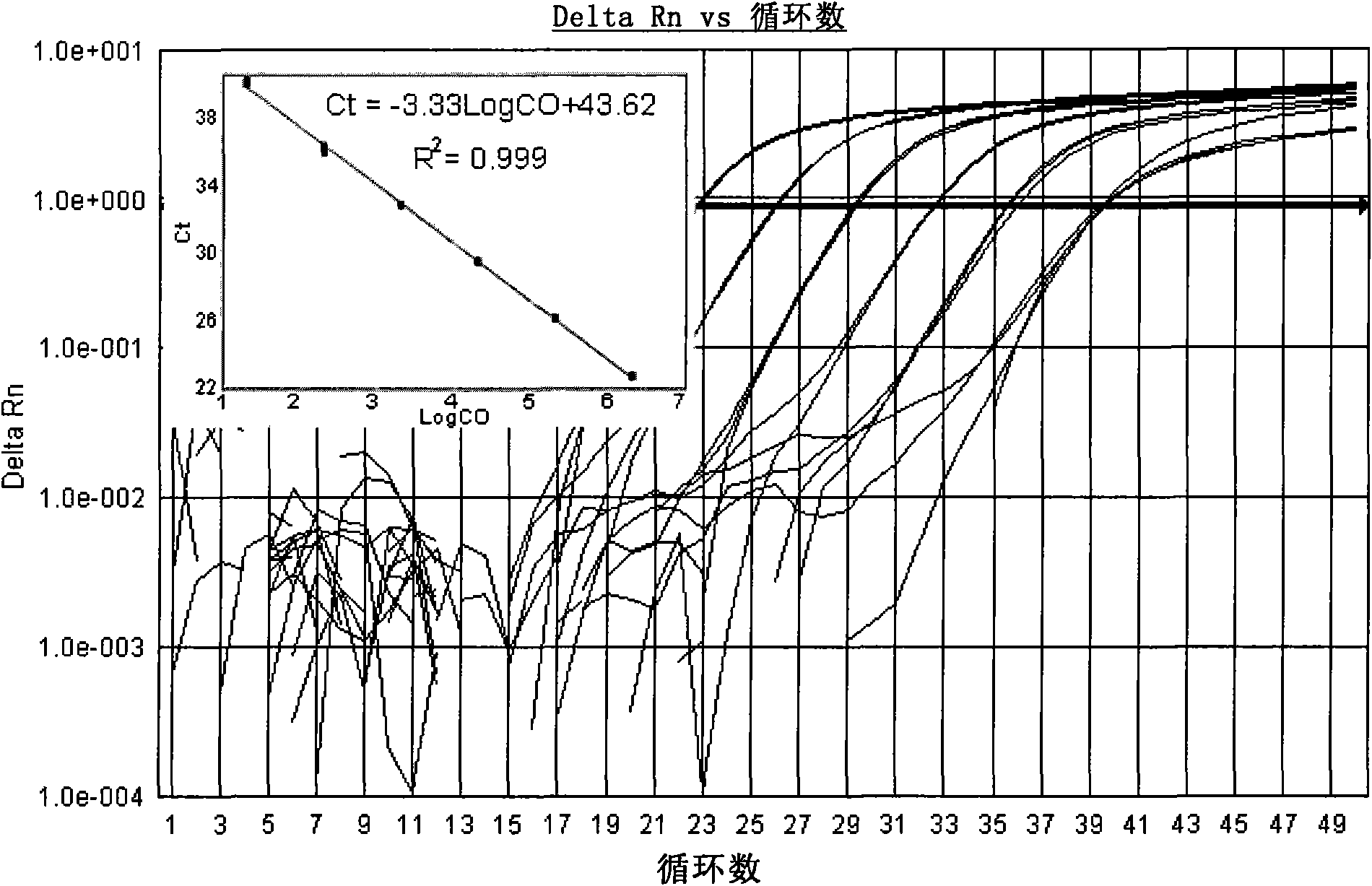
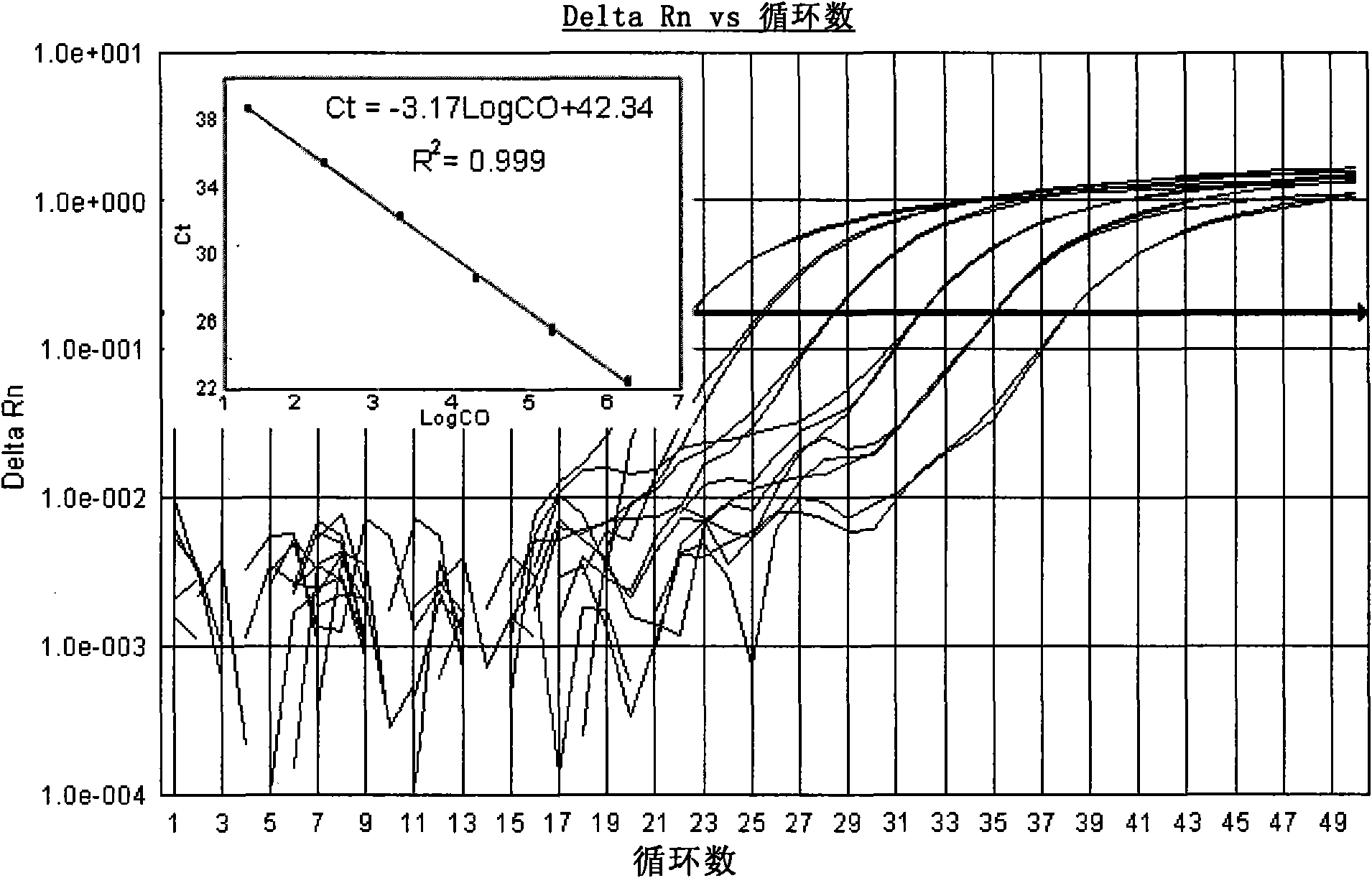
[0122] Search for the cDNA sequences of Coxsackieviruses A9, A16, B2, B3 and B5 in the GenBank database, compare and analyze them, and find out that each serotype is relatively conserved, but it is different...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com