Method for synthesizing 7-hydroxy-4-methylcoumarin with solvent-free catalysis of ionic liquid
A technology of methyl coumarin and ionic liquids, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve complex operations, high costs, and reaction conditions Harsh and other problems, to achieve the effect of simple preparation process, less corrosion, and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
[0032] Example 1-2: The effect of catalyst type on yield
[0033] Reaction conditions: Put 11.01g (0.1mol) of resorcinol and 14.32g (0.11mol) of ethyl acetoacetate into a three-necked flask. The amount of catalyst accounts for 3% (0.33g) of the amount of resorcinol. Investigate the reaction of the catalyst The results are shown in Table 1:
[0034] Table 1 The influence of the catalyst on the reaction
[0035] Serial number
[0036] The type of catalyst has a great influence on the product yield. It can be seen from Table 1 that in the absence of a catalyst, the synthesis reaction of resorcinol and ethyl acetoacetate does not occur. The catalytic efficiency of sulfamic acid is the lowest, and the catalytic activity of the N-methylpyrrolidone hydrogen sulfate ionic liquid catalyst is the highest and the yield is the highest.
Embodiment 3-7
[0037] Example 3-7: The influence of the amount of catalyst on the yield
[0038] Reaction conditions: Add 11.01g (0.1mol) of resorcinol and 13.02g (0.1mol) of ethyl acetoacetate into a three-necked flask, the reaction time is 2.5h, the catalyst is N-methylpyrrolidone hydrogensulfate, and different dosages are used to obtain The results are shown in Table 2:
[0039] Table 2 The influence of the amount of catalyst on the reaction
[0040]
[0041] Theoretically, as the amount of catalyst increases, the reaction speed increases. However, considering the reaction time and product purity, the amount of catalyst should not be too large. It can be seen from Table 2 that the amount of N-methylpyrrolidone bisulfate catalyst as a percentage of the amount of resorcinol has a certain influence on the reaction. That is, within a certain range, the yield varies with the decrease of the catalyst. When it increases and reaches the maximum value, the yield will decrease with the decrease of the ...
Embodiment 8
[0042] Embodiment 8: The best embodiment
[0043] The molar ratio of resorcinol and ethyl acetoacetate is 1:1.1, the catalyst is N-methylpyrrolidone hydrogen sulfate, the amount is 3% of the amount of resorcinol, and the reaction reflux time is 2.5h. The yield obtained under this improved synthesis process can reach 69.60%.
[0044] a. The product structure identification and analysis of Example 8 was carried out by infrared spectrum analysis method, and the specific analysis conditions were:
[0045] Instrument: Shimadzu Fourier Transform Infrared Spectrometer IRAffinity-1; Carrier: KBr
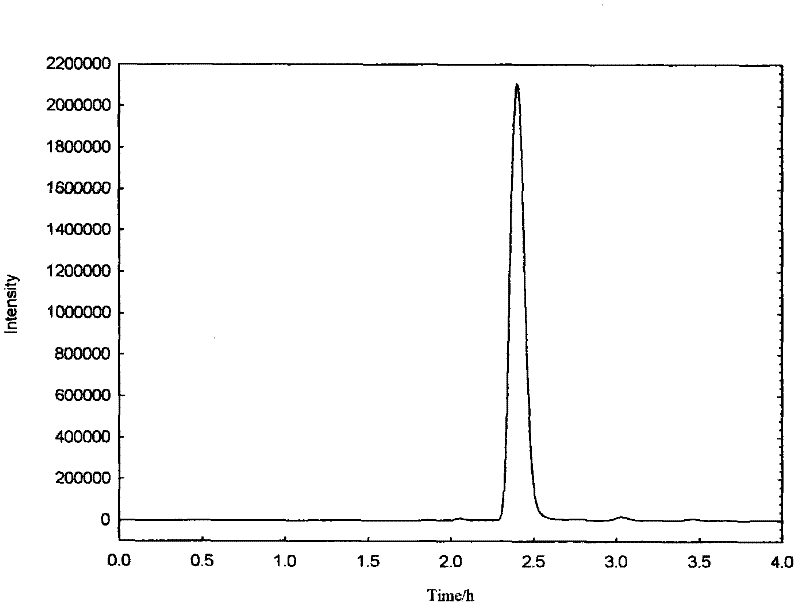
[0046] FT-IRumax of the product: (KBr) cm: 3157cm -1 (Ar-H), 1677cm -1 (C=O), 1608cm -1 , 1450cm -1 Is the skeleton vibration of the benzene ring, 1388cm -1 (CH 3 ), 1068cm -1 (C-O), 844cm -1 Corresponding to the C=O-H out-of-plane bending vibration outside the benzene ring, (such as figure 1 Shown).
[0047] Melting point: The synthetic 7-hydroxy-4-methylcoumarin has a melting point of 188℃-192℃.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com