Irbesartan sodium micro composite powder and tablets and preparation method thereof
A composite powder and irbesartan technology, which is applied in pharmaceutical formulation, powder delivery, pill delivery, etc., can solve the problems of insufficient improvement of irbesartan bioavailability, uneven distribution, large drug particles, etc. , to achieve the effect of good application prospect, simple process and high dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
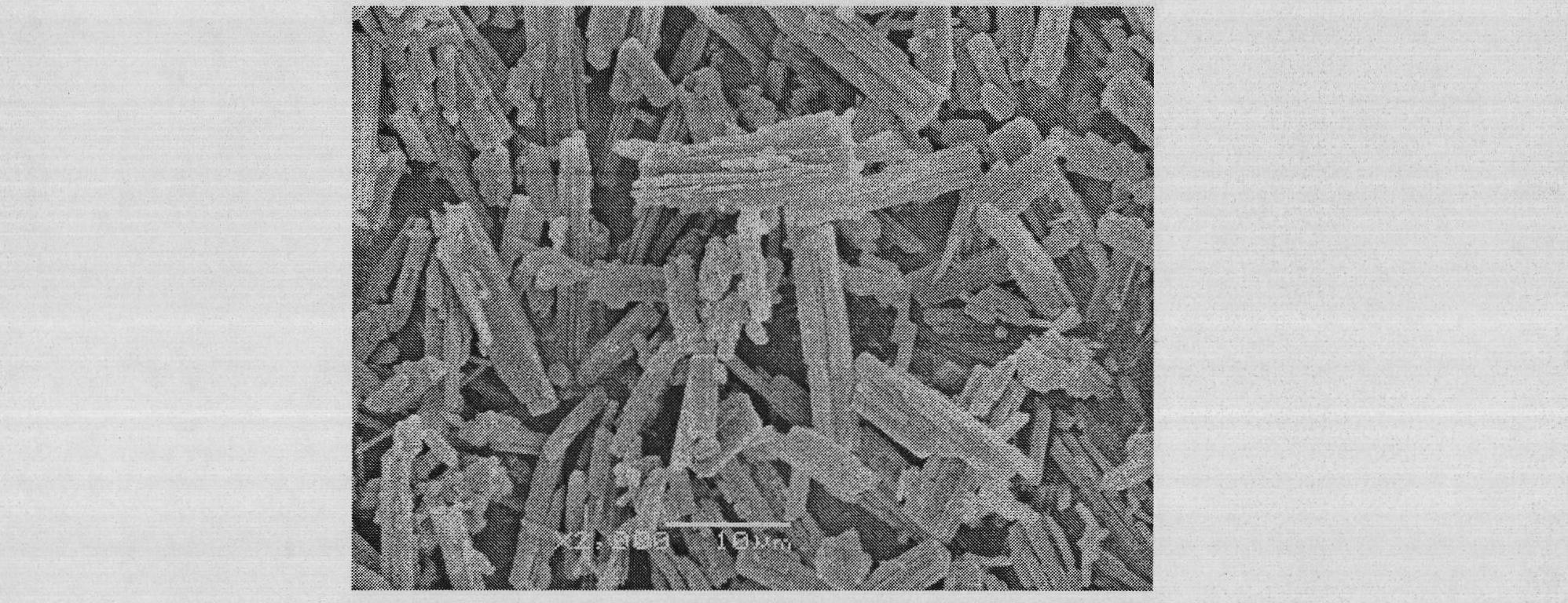
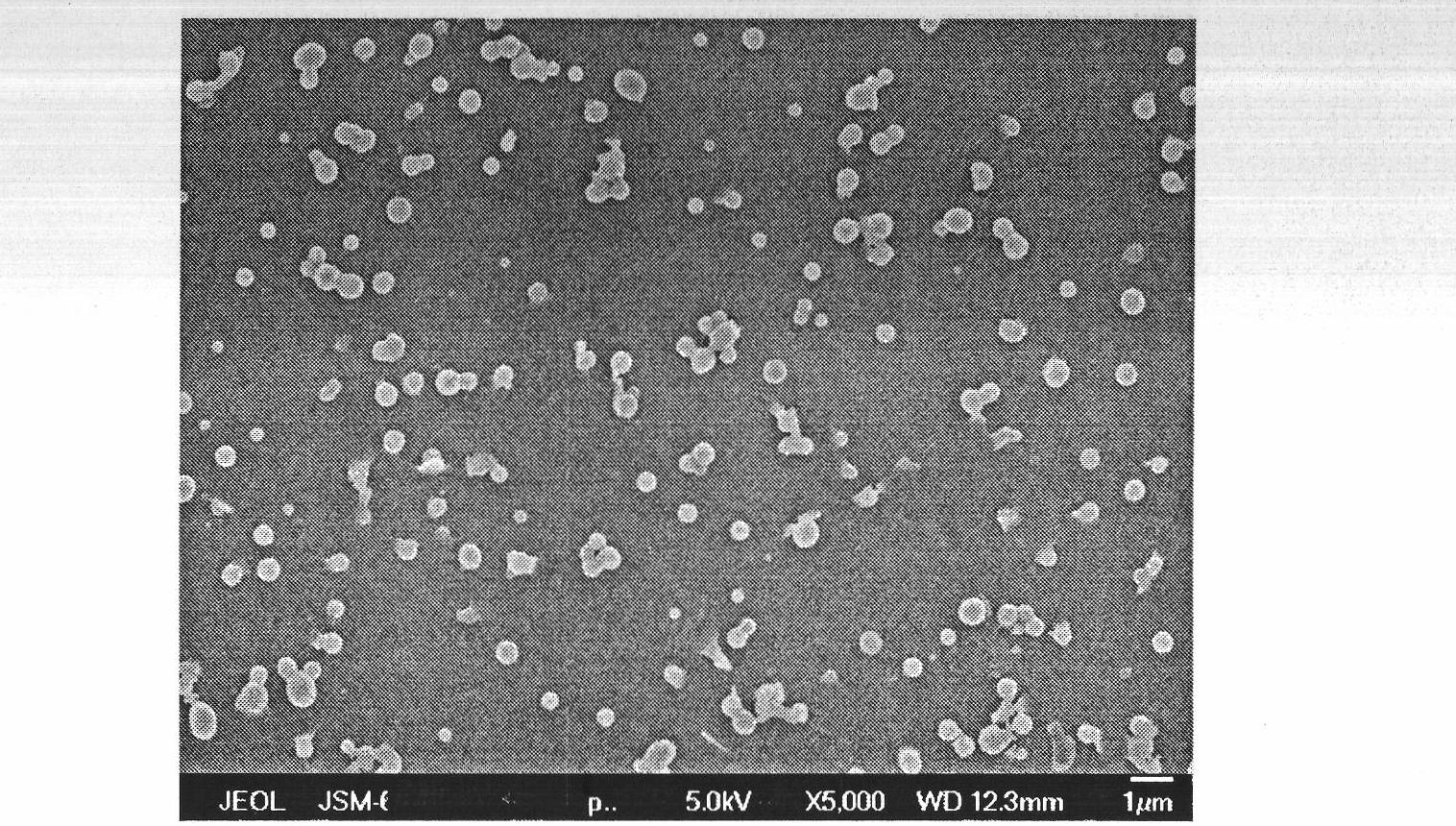
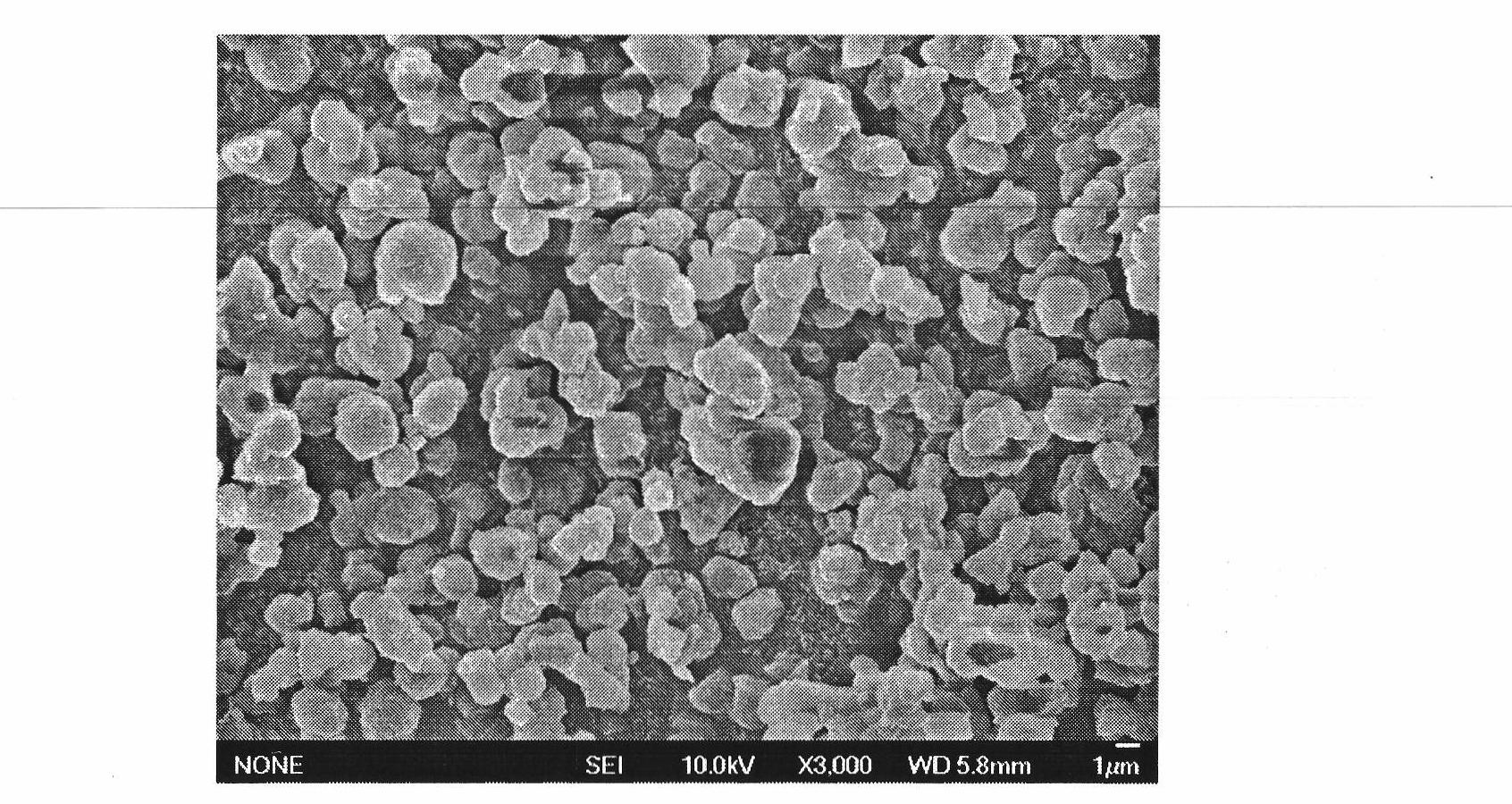
[0057] A: Weigh 5g of irbesartan raw material drug and dissolve it in 100mL of tetrahydrofuran; weigh 0.5g of mannitol and dissolve it in 1.5L of water. Under the condition of controlling the system temperature at 10°C, mix and stir the drug solution and aqueous solution to obtain drug slurry material, wherein the average particle size of the drug particles is 600nm;
[0058] B: Weigh 0.5g of microcrystalline cellulose, 0.5g of starch, and 0.3g of cross-linked polyvinylpyrrolidone and disperse them in the slurry obtained in step A;
[0059] C: Spray-dry the slurry obtained in step B to obtain amorphous irbesartan micro-composite powder, in which the mass of irbesartan is 73%; the spray-drying conditions are: feed flow rate is 30mL / min, inlet temperature 130°C, compressed air pressure is 0.5MPa.
[0060] D: Weigh 1.5 g of the composite powder obtained in step C, add an appropriate amount of 4% PVP ethanol-water solution, granulate, and dry at 70°C to obtain dry granules. The v...
Embodiment 2
[0063] A: Weigh 6g of irbesartan raw material drug and dissolve it in 600mL of methanol; weigh 0.8g of lactose and dissolve it in 6L of water, and mix and stir the drug solution and aqueous solution under the condition of controlling the system temperature at 15°C to obtain drug slurry , wherein the average particle size of the drug particles is 400nm;
[0064] B: Weigh 0.8g of microcrystalline cellulose and 0.7g of carboxymethyl starch and nano-disperse them in the slurry obtained in step A;
[0065] C: Spray-dry the slurry obtained in step B to obtain amorphous irbesartan micro-composite powder, in which the mass of irbesartan is 72%; the spray-drying conditions are: feed flow rate is 20mL / min, inlet temperature 120°C, compressed air pressure is 0.6MPa.
[0066] D: Weigh 0.15g lactose, 0.15g microcrystalline cellulose, 0.08g sodium carboxymethyl starch, mix evenly with 2g of the composite powder obtained in step C, add an appropriate amount of 2% PVP ethanol-water solution,...
Embodiment 3
[0069] A: Weigh 4g of irbesartan raw material and dissolve it in 300mL N,N-dimethylformamide; weigh 1g of polyethylene glycol and 0.2g of sodium lauryl sulfate and dissolve it in 1.5L of water. Under the condition of temperature of 25°C, the drug solution and the aqueous solution are mixed and stirred to obtain a drug slurry, wherein the average particle size of the drug particles is 900nm;
[0070] B: Weigh 0.8g lactose, 0.7g starch and disperse in the slurry obtained in step A;
[0071] C: Spray-dry the slurry obtained in step B to obtain amorphous irbesartan micro-composite powder, in which the mass of irbesartan is 59%; the spray-drying conditions are: feed flow rate is 15mL / min, inlet temperature 165°C, compressed air pressure is 0.7MPa.
[0072] D: Weigh 0.1g lactose, 0.1g starch, 0.2g croscarmellose sodium, mix evenly with 2g of the composite powder obtained in step C, add an appropriate amount of 3% PVP ethanol-water solution, granulate, and heat at 55°C Dried to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com