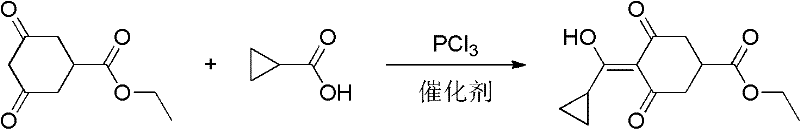
The preparation method of trinexapac-ethyl
A technology of trinexapac-ethyl and ethyl dioxetate, applied in the field of preparation of trinexapac-ethyl, can solve the problems of low yield, increased cost, unfavorable environmental protection and the like, and achieves simple process, high yield and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In a three-necked reaction flask equipped with a stirring motor, a condenser, and a dropping funnel, add 36.8 grams of ethyl 3,5-dioxcyclohexyl carboxylate, 17.2 grams of cyclopropanecarboxylic acid, 100 grams of chloroform, and N, N-dimethyl With 25 grams of aniline, start to drop 13.8 grams of phosphorus trichloride under cooling to 0° C. and stirring. After the dropwise addition of phosphorus trichloride, add 7 grams of 4-dimethylaminopyridine, react at 60° C. for 4 hours, and cool to 0 ℃, add 100ml of water, adjust the pH to 5-6, separate the water layer, remove chloroform and N, N-dimethylaniline, and distill under reduced pressure to obtain the target product 4-cyclopropyl (hydroxyl) methylene 3,5-diox 37 grams of ethyl cyclohexanecarboxylate is trinexapac-ethyl, the content is 96%, and the yield is 70.5%.
Embodiment 2
[0020] In a three-necked reaction flask equipped with a stirring motor, a condenser, and a dropping funnel, add 36.8 grams of ethyl 3,5-dioxcyclohexyl carboxylate, 17.2 grams of cyclopropanecarboxylic acid, 100 grams of toluene, and 25 grams of triethylamine. Add 13.8 grams of phosphorus trichloride dropwise under cooling and stirring, and the temperature rises to 30°C. After dropping the phosphorus trichloride in 1 hour, add 5 grams of 4-dimethylaminopyridine, keep the reaction at 60°C for 5 hours, and add 100 grams of water Finally, adjust the pH to 5-6, separate the water layer, remove the toluene, and distill under reduced pressure to obtain 31 g of trinexapac-ethyl, with a content of 91% and a yield of 56.0%.
Embodiment 3
[0022] In a three-necked reaction flask equipped with a stirring motor, a condenser, and a dropping funnel, add 36.8 grams of ethyl 3,5-dioxcyclohexyl carboxylate, 17.2 grams of cyclopropanecarboxylic acid, and 100 grams of N, N-dimethylaniline, Add 13.8 grams of phosphorus trichloride dropwise under cooling and stirring. After dropping the phosphorus trichloride, add 7 grams of 4-dimethylaminopyridine, keep the reaction at 60°C for 4 hours, add 100 grams of water, adjust the pH to 5-6, and separate the water layer, removed N,N-dimethylaniline, and distilled under reduced pressure to obtain 39 grams of trinexapac-ethyl, with a content of 97.5% and a yield of 75.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com