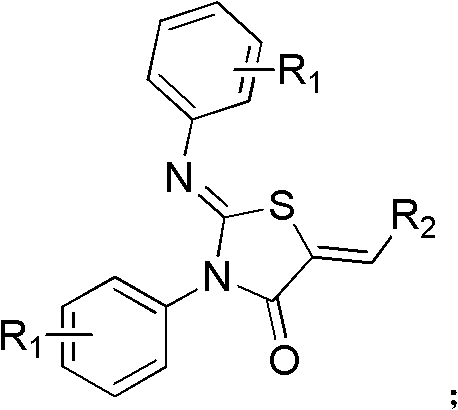
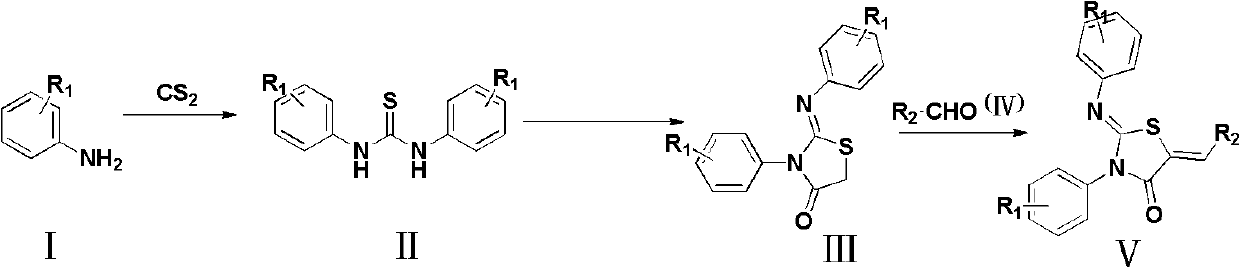
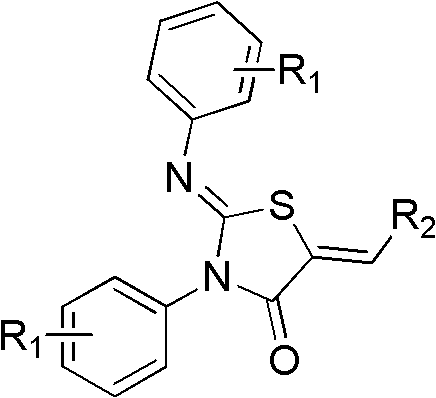
A kind of 4-thiazolidinone carboxylic acid derivative and its preparation method and application
A technology of thiazolidinone carboxylic acid and derivatives, which is applied in the field of medicinal chemistry and combinatorial chemistry, can solve the problems of few synthesis reports, and achieve good anti-biofilm activity, good inhibitory effect, and good antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of 2-(4-chlorophenylimino)-3-(4-chlorophenyl)-5-[4-(4-vinylphenoxymethyl)]benzoic acid-4-thiazolidinone
[0031] Preparation of 1,3-bis(4-chlorophenyl)thiourea
[0032] 4-Chloroaniline (20mmol), CS 2 (10mmol) and 6mol / L aqueous NaOH solution (20ml) were placed in a 100ml flask and heated to 55°C, and the reaction was detected by TLC. After reacting for 8 hours, the system was cooled to room temperature, poured into 100ml of ice-water mixture, stirred, and filtered. The filter cake was washed with water several times, and recrystallized from methanol to obtain a white powder, which was 1,3-bis(4-chlorophenyl)thiourea.
[0033] Preparation of 2-(4-chlorophenylimino)-3-(4-chlorophenyl)-4-thiazolidinone
[0034] 1,3-bis(4-chlorophenyl)thiourea (3mmol), ethyl bromoacetate (3.6mmol), anhydrous sodium acetate (3mmol) and ethanol (20ml) were added to a 50ml flask and refluxed for 1 hour. After the reaction, the solvent was evaporated to dryness under reduced pre...
Embodiment 2
[0041] Preparation of 2-(4-chlorophenylimino)-3-(4-chlorophenyl)-5-[3-(5-vinylfuryl)]benzoic acid-4-thiazolidinone
[0042] Acetic acid 2ml, alanine 0.03g, 3-(5-formyl-2-furyl)-benzoic acid (0.41mmol) and 2-(4-chlorophenylimino)-3-(4-chlorophenyl )-4-thiazolidinone (0.41mmol) (preparation method is the same as the preparation of 2-(4-chlorophenylimino)-3-(4-chlorophenyl)-4-thiazolidinone in Example 1) The flask was refluxed for 0.5 hours. Cool to room temperature, add ice water and let stand, filter with suction, recrystallize with methanol to obtain a yellow powder, which is 2-(4-chlorophenylimino)-3-(4-chlorophenyl)-5-[ 3-(5-Vinylfuryl)]benzoic acid-4-thiazolidinone.
[0043] Yellow powder, yield 81%; 1 H NMR (300MHz, DMSO): δ (ppm) = 12.79 (s, 1H), 8.28 (s, 1H), 7.93 (d, J = 7.8Hz, 1H), 7.82 (d, J = 7.9Hz, 1H) , 7.67(s, 1H), 7.61(d, J=2.9Hz, 4H), 7.49(d, J=8.8Hz, 3H), 7.35(d, J=3.7Hz, 1H), 7.18(d, J= 3.7Hz, 1H), 7.06(d, J=8.6Hz, 2H).
[0044] ESI-MS: m / z 535.1 (M+1) ...
Embodiment 3
[0046] Preparation of 2-(4-chlorophenylimino)-3-(4-chlorophenyl)-5-(4-vinylphenoxyacetic acid)-4-thiazolidinone
[0047] 2ml of ethanol, 0.03g of proline, 2-(4-formyl)phenoxyacetic acid (0.37mmol) and 2-(4-chlorophenylimino)-3-(4-chlorophenyl)-4-thiazole Alkanone (0.37mmol) (preparation method is the same as the preparation of 2-(4-chlorophenylimino)-3-(4-chlorophenyl)-4-thiazolidinone in Example 1) was added to a 25ml flask, and reflux reaction 1.5 hours. Cool to room temperature, add ice water and let it stand, suction filter, recrystallize with methanol to obtain a white powder, which is 2-(4-chlorophenylimino)-3-(4-chlorophenyl)-5-( 4-vinylphenoxyacetic acid)-4-thiazolidinone.
[0048] White powder, yield 39%; 1 H NMR (300MHz, DMSO): δ (ppm) = 13.11 (s, 1H), 7.79 (s, 1H), 7.61 (d, J = 1.2Hz, 4H), 7.54 (d, J = 8.8Hz, 2H) , 7.44(d, J=8.6Hz, 2H), 7.03(dd, J=14.0, 8.7Hz, 4H), 4.74(s, 2H).
[0049] ESI-MS: m / z 498.9 (M+1) +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com