A kind of ambroxol hydrochloride sustained-release dry suspension and preparation method thereof
A technology of ambroxol hydrochloride and dry suspension, which is applied in the field of ambroxol hydrochloride slow-release dry suspension and its preparation, can solve the problems of low frequency of bioavailability, poor drug compliance, etc., and solve the pain of taking medicine , blood drug concentration is stable, and the effect of improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
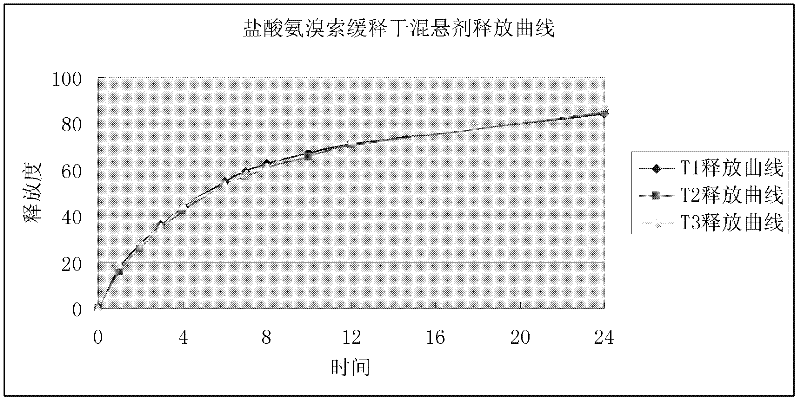
Image
Examples
Embodiment 1
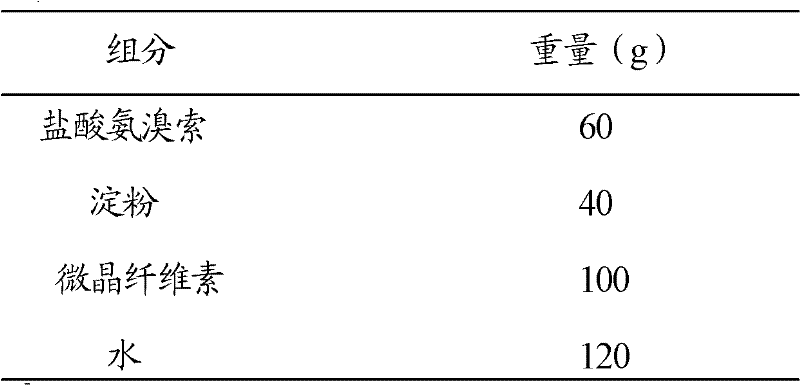
[0030] 1. Preparation of drug-loaded pellet core
[0031] The proportioning of each component of drug-loaded pellet core is as follows:
[0032]
[0033] In other embodiments, the ratio of broxol hydrochloride, starch, and microcrystalline cellulose can also be: broxol hydrochloride 20g, starch 80g, microcrystalline cellulose 100g; or broxol hydrochloride 50g, starch 50g, microcrystalline cellulose 100g.
[0034] making process:
[0035] Ambroxol hydrochloride, starch, and microcrystalline cellulose are mixed uniformly by an equal increase method, and the aqueous solution is used as a binder to make a soft material, and the extrusion-spheronization mechanism pellets are adopted, and the particle size of the sieve plate is 0.2mm, and then Dried in an oven at 50°C, sieved, and selected pellets smaller than 80 mesh for the next step of coating process.
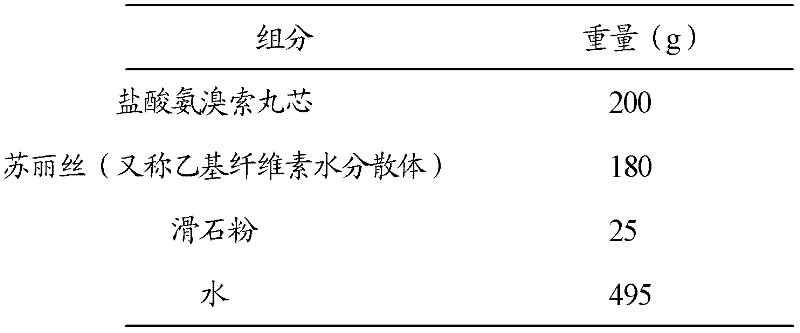
[0036] 2. Packed with slow-release coating
[0037]
[0038] Coating process: Add the prescribed amount of Surelease ...
Embodiment 2
[0044] 1. Preparation of drug-loaded pellet core
[0045] The proportioning of each component in the drug-loaded pellet core is as follows:
[0046]
[0047] Note: The above percentages are percentages by weight.
[0048] The preparation process of drug-loaded pellet core is as follows:
[0049] Mix ambroxol hydrochloride, lactose, and microcrystalline cellulose evenly by increasing the amount in equal amounts, put them into a multifunctional fluidized bed, and make pellets by centrifugal method. The liquid speed is properly adjusted depending on the degree of dryness and wetness of the drug powder, so that the drug powder gradually forms fine pellets under the action of centrifugal force and binder, dried in an oven at 50°C, and sieved to select pellets smaller than 80 mesh. Perform coating.
[0050] The process parameters set above are: the speed of the turntable is 300r / min, the air intake volume can be set in the range of 20HZ~30HZ, in this embodiment it is 27HZ, in ...
Embodiment 3
[0060] 1. Preparation of drug-loaded pellet core
[0061]
[0062] making process:
[0063] Ambroxol hydrochloride, lactose, and microcrystalline cellulose are mixed uniformly by an equal increase method, and an aqueous solution is used as a binder to make a soft material, and extrusion-spheronization mechanism pellets are used, and the particle size of the sieve plate is 0.2mm. Dried in an oven at 50°C, sieved, and selected pellets smaller than 80 mesh for coating.
[0064] 2. Packed with slow-release coating
[0065]
[0066] Add the prescribed amount of talc powder into water and stir for 30 minutes, add triethyl citrate, Eudragit RS30D, RL30D water dispersion to make it fully dispersed, continue stirring for 30 minutes to form a slow-release coating solution, and set aside. The drug-loaded pellet core was placed in a fluidized bed, and various process parameters were set. During the experiment, the parameters were constantly adjusted to ensure the state of fluidiza...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com