Fusogenic peptide containing thrombin fragment
A fusion peptide and thrombin technology, applied in the treatment of diseases of skin tissue damage and corneal damage, and in the field of preparation of drugs for the treatment of skin tissue damage and corneal damage, can solve the problems of short half-life and small molecular weight of TP508
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
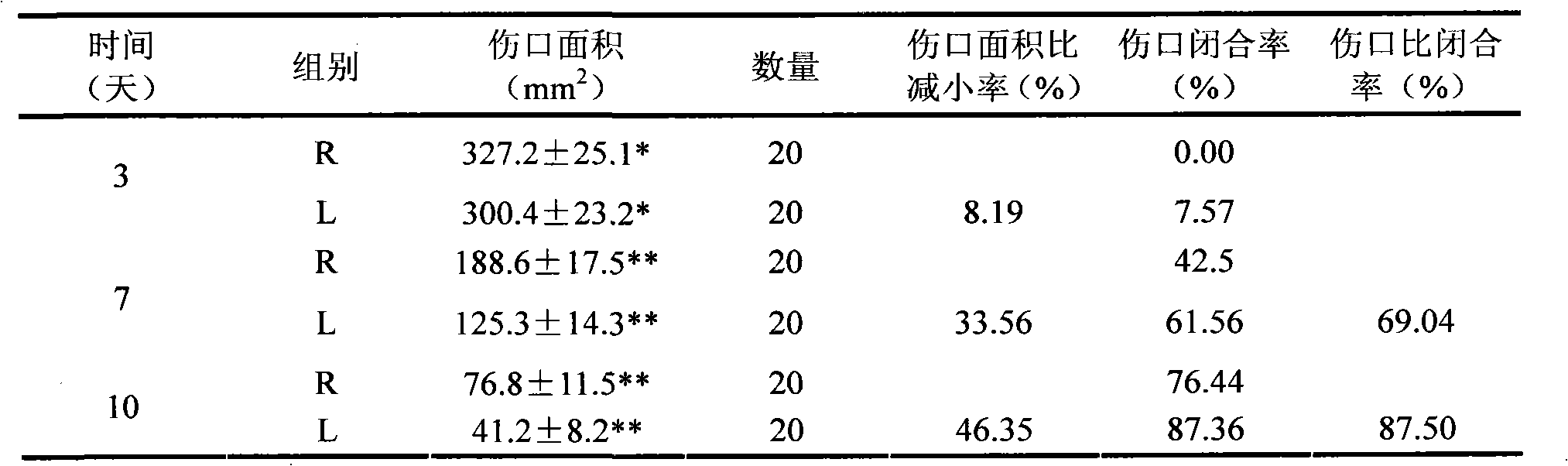
Image
Examples
example 1
[0030] Construction of Example 1 Recombinant Fusion Protein Particle and Acquisition of Engineering Bacteria
[0031] Using the designed amino acid sequence of SEQ ID NO1 as a template, according to the central method, select the codon preferred by Escherichia coli to obtain the corresponding nucleic acid sequence of the fusion protein, and then use the whole gene synthesis technology to obtain the above-mentioned target gene fragment. The recombinant gene vector pBSK-S-TP508 was obtained through the recombination technique known to those skilled in the art, and the sequence of the gene fragment inserted into the vector was determined. After verifying the correctness of its gene sequence, it was amplified and stored, and the target gene fragment was cut out from the vector, inserted into the expression vector, and the recombinant plasmid was named pS-TP508. Then use the CaCl2 method to transform Escherichia coli BL21, spread it on the surface of the screening plate medium cont...
example 2
[0032] Example 2 Expression of the protein of interest
[0033] 1) Shake flask expression: the genetically engineered bacterium pS-TP508 / BL21 prepared as above was cultured in LB medium overnight in a shake flask (37°C, 200rpm), and then inoculated into LB medium at a volume ratio of 1:30 After culturing at 37°C for 3 hours, 0.1 mM IPTG was added for induction for 4 hours. The collected bacteria were analyzed by SDS-PAGE electrophoresis, and it was found that the fusion protein (43KD) was mainly expressed in soluble form, and the expression amount accounted for 40% of the total protein of the bacteria.
[0034] 2) Large-scale fermentation expression:
[0035] a. Medium composition:
[0036] a) Seed liquid medium (LB):
[0037] Tryptone: 10 g / L, yeast powder: 5 g / L, sodium chloride: 10 g / L.
[0038] b) Tank culture medium (15 liters):
[0039] Disodium hydrogen phosphate: 250 grams, potassium dihydrogen phosphate: 110 grams, sodium chloride: 13. grams, ammonium chloride: 3...
example 3
[0045] Example three purpose protein purification
[0046] Recombinant protein expression was accomplished by S-TP508 / JM109 engineering bacteria. After fermentation, the fermentation liquid was collected by centrifugation, the bacteria were collected by pressure homogenization, and the supernatant was collected and put on a Glutathione Sepharose chromatography column. After equilibration, the fusion protein was washed with an eluent containing reduced glutathione (GSH), and thrombin was added. (5NIHU / mL) enzyme digestion at 37°C for 2 hours, and then remove the GST protein by cation exchange chromatography (SP Sepharose Fast Flow) to obtain recombinant S-TP508 with a purity greater than 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com