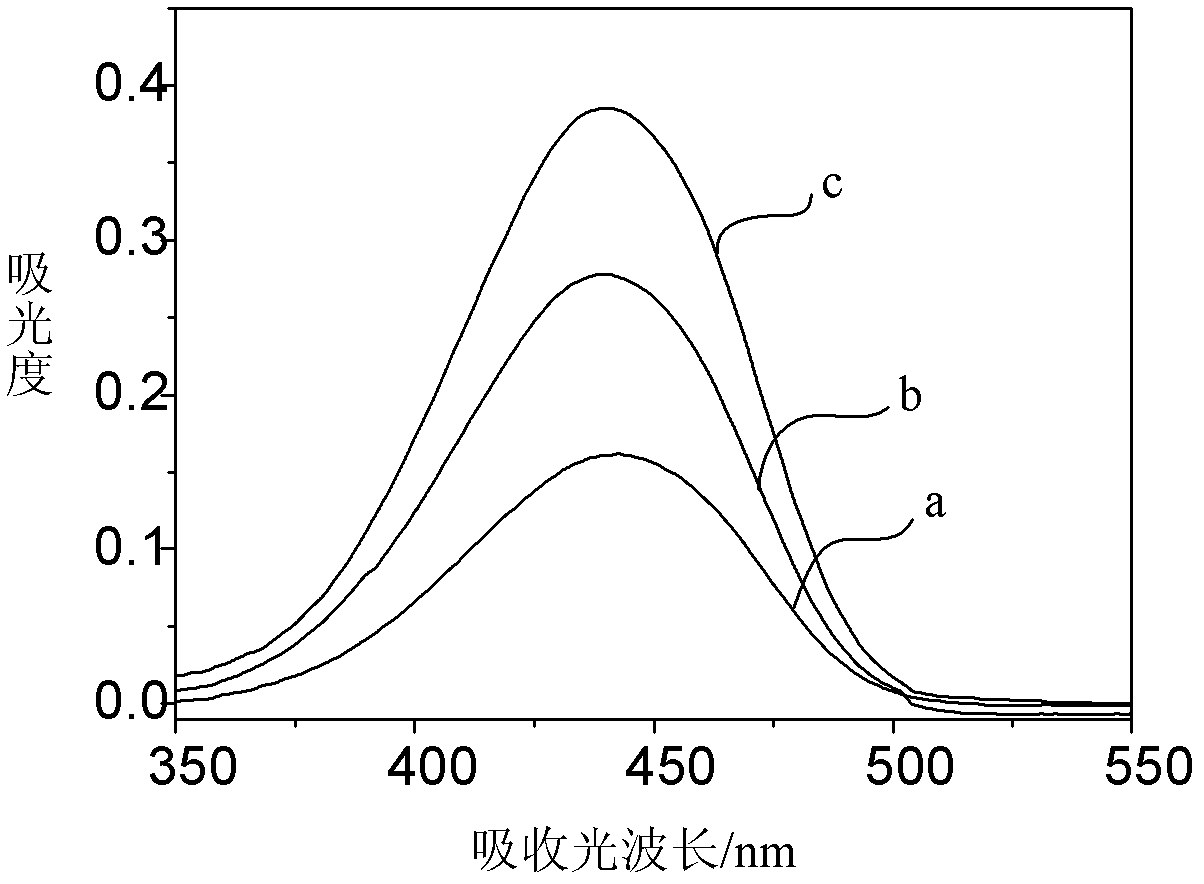
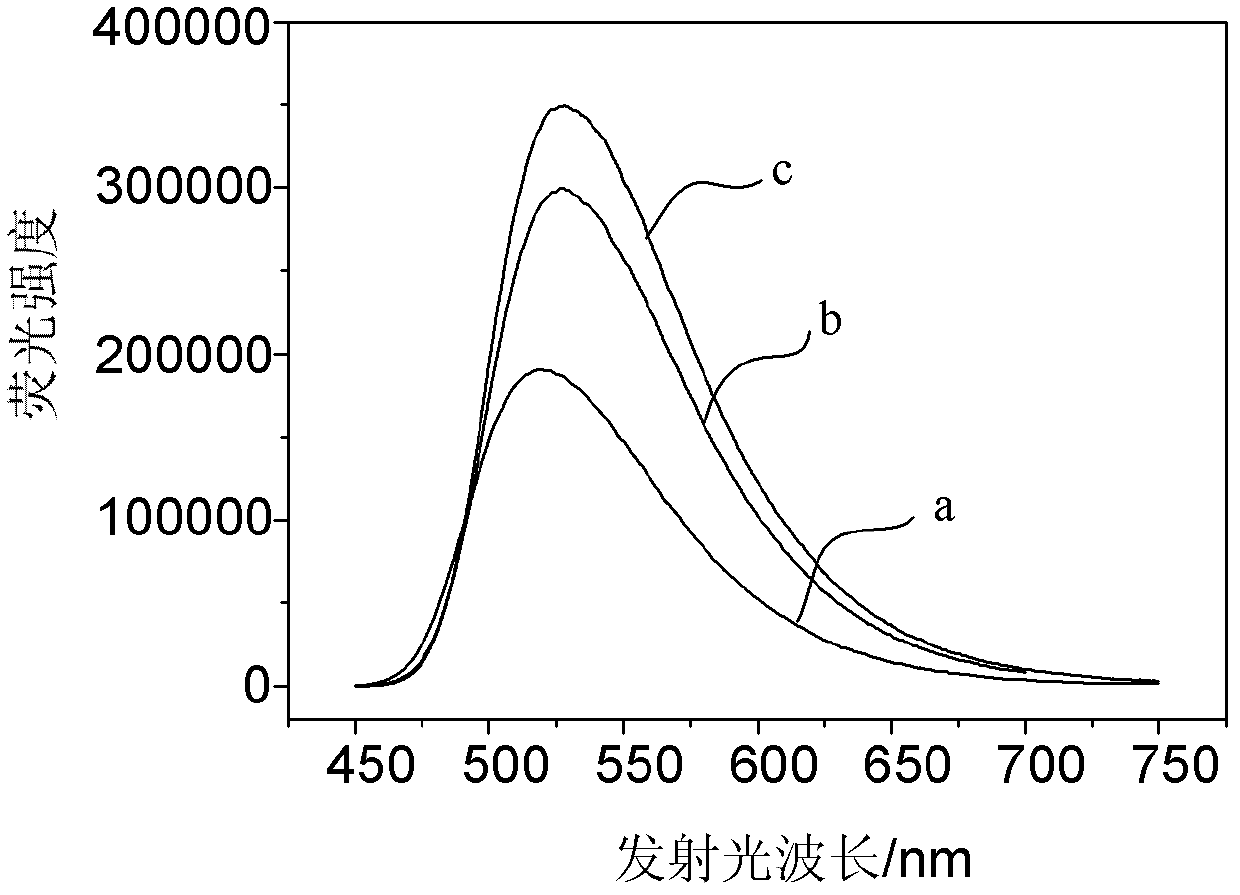
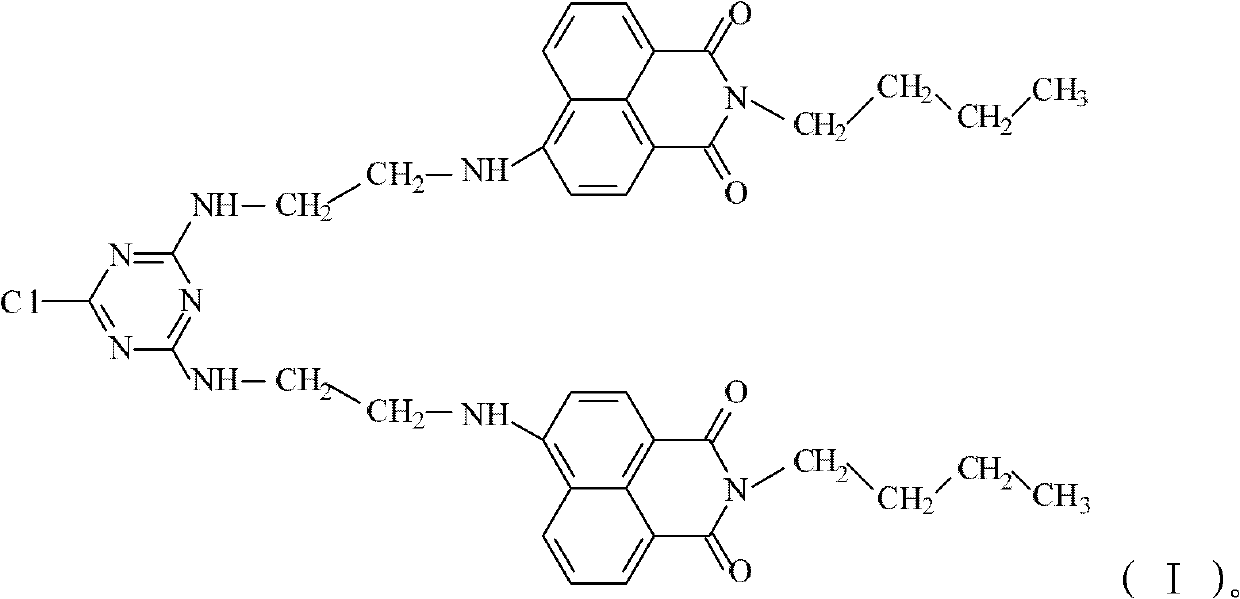
1,8-naphthalimide derivatives and preparation method thereof
A technology of naphthalimide and derivatives, applied in the field of fluorescent materials, which can solve the problems of low utilization efficiency and mutual interference of fluorophores
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1 prepares EBNP
[0062] Add N-n-butyl-4-bromo-1,8-naphthalimide and ethylenediamine solution to ethylenediamine, the added N-n-butyl-4-bromo-1,8-naphthalimide The molar ratio to ethylenediamine was 1:45, and the mixed solution was stirred and heated at 70°C for 10 hours, then cooled, and water was added to obtain a yellow precipitate.
[0063] The mixture obtained after the reaction was filtered, washed with water, dried in vacuo, and recrystallized from toluene to obtain EBNP as a yellow solid with a yield of 88.0%.
[0064] The EBNPs used in the following examples were all prepared in this example.
Embodiment 2
[0066] Add DIPEA, EBNP and CNC to anhydrous tetrahydrofuran, and the molar ratio of reactants is as follows: n(EBNP):n(CNC):n(DIPEA)=1.8:1.0:2.1. The above mixed solution was placed in an ice-water bath (0-2°C) for 4 h, and then under N 2 Under protection, the temperature was raised to 66°C and the reaction was refluxed for 48h. After natural cooling, the solvent was removed under reduced pressure, and the filtrate was washed with deionized water until the filtrate was colorless, then washed with ethanol and petroleum ether in turn, and dried in vacuum to obtain BCBNT with a yield of 49.6%.
[0067] The product obtained in this example is subjected to infrared spectrum analysis, nuclear magnetic resonance analysis, mass spectrometry and elemental analysis successively, and the analysis results are as follows:
[0068] Infrared spectrum analysis results: 3364.9cm-1 is the stretching vibration of -NH-, 2955.9cm-1 and 2871.0cm-1 are the stretching vibrations of methylene, 1682.5...
Embodiment 3
[0077] Add DIPEA, EBNP and CNC to anhydrous tetrahydrofuran, and the molar ratio of reactants is as follows: n(EBNP):n(CNC):n(DIPEA)=2.2:1.0:2.1. The above mixed solution was placed in an ice-water bath (2-3°C) for 4 h, and the 2 Under protection, the temperature was raised to 65°C and the reaction was refluxed for 48h. After natural cooling, the solvent was removed under reduced pressure, and the filtrate was washed with deionized water until the filtrate was colorless, then washed with ethanol and petroleum ether in turn, and dried in vacuum to obtain BCBNT with a yield of 87.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com