Novel inhalant containing glucocorticoid and bronchodilator
A technology of glucocorticoids and agonists, which is applied in the direction of respiratory diseases, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of reducing lung deposition rate, complex mixing process, and uniformity impact, etc., to achieve Improve the lung deposition rate, simplify the mixing process, and easy to mix evenly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
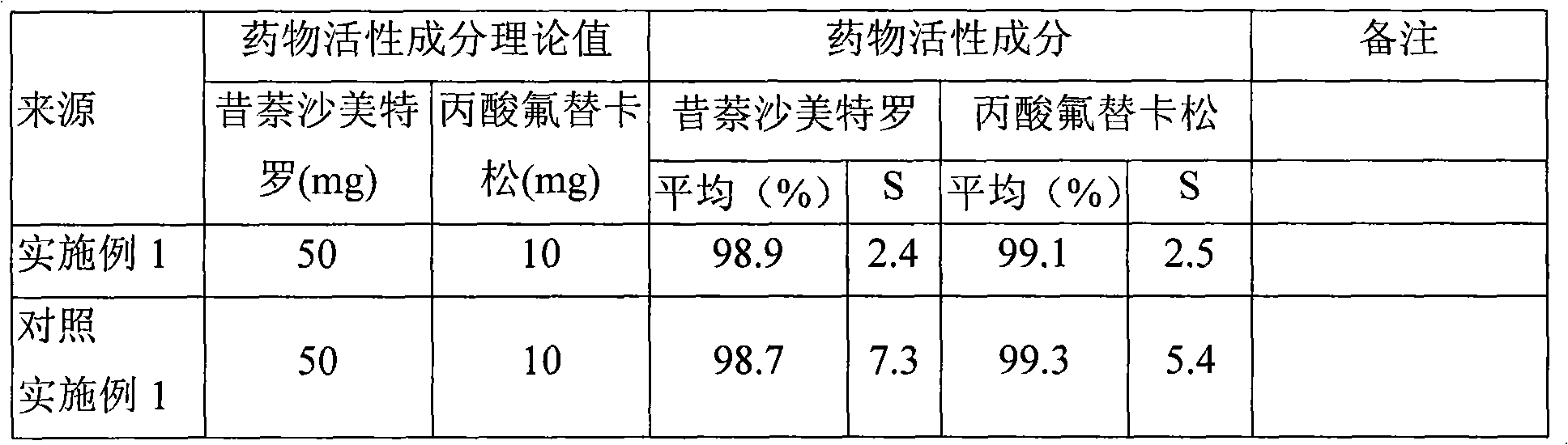
Embodiment 1
[0065] The preparation of comparative example 1 fluticasone propionate, xinasalmeterol granules
[0066] Prepare 100 ml of methanol solution of fluticasone propionate at 5 mg / ml and spray-dry to obtain dry powder particles with an average particle size of 3.16 μm for future use. The operating parameters of spray drying are: feed rate 10ml / min, inlet temperature 85°C, additional air 0.55MPa.
[0067] Prepare 100 ml of methanol solution of xinasalmeterol at 1 mg / ml and spray-dry to obtain dry powder particles with an average particle size of 2.79 μm, which is ready for use. The operating parameters of spray drying are: feed rate 10ml / min, inlet temperature 85°C, additional air 0.55MPa.
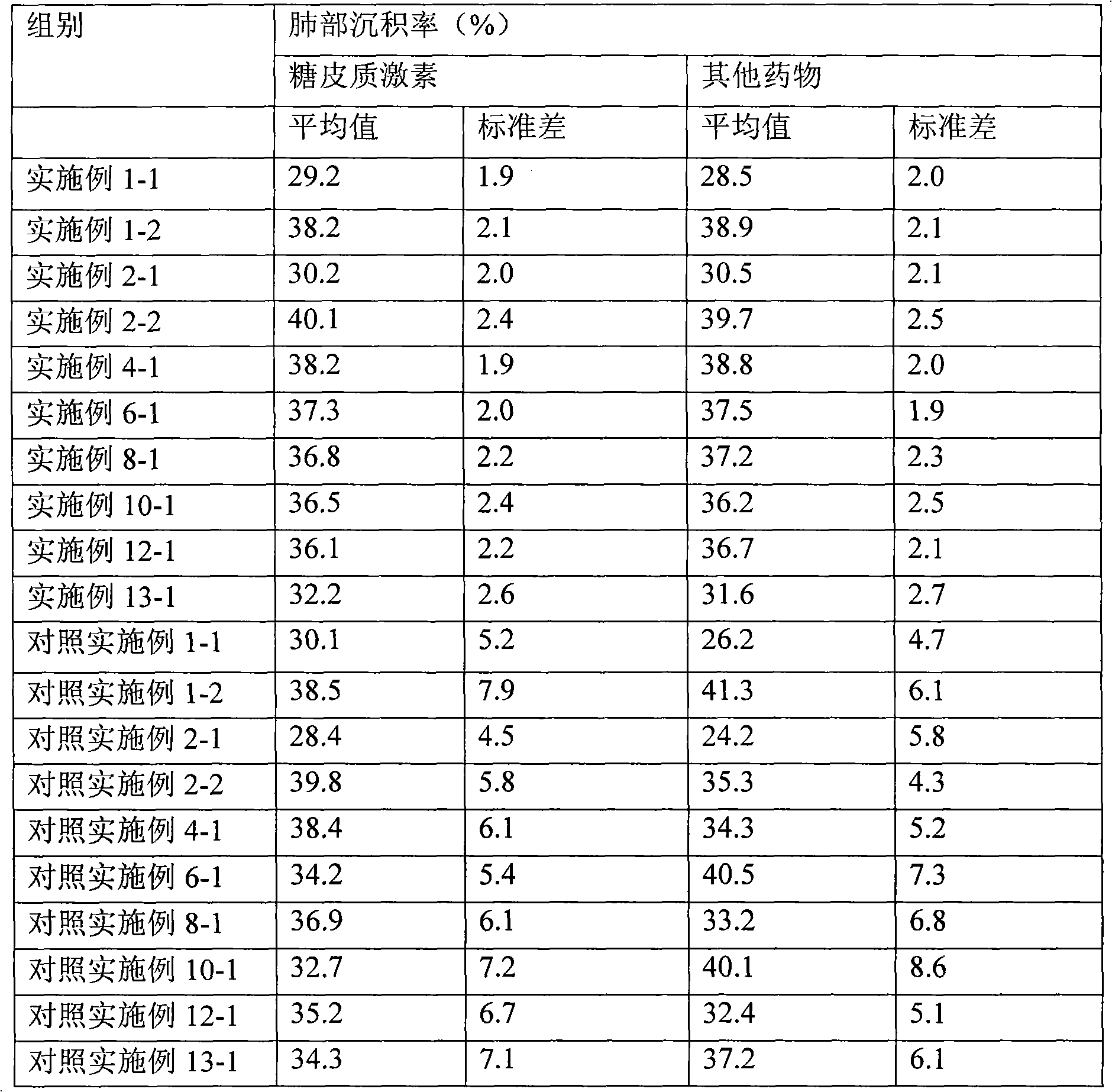
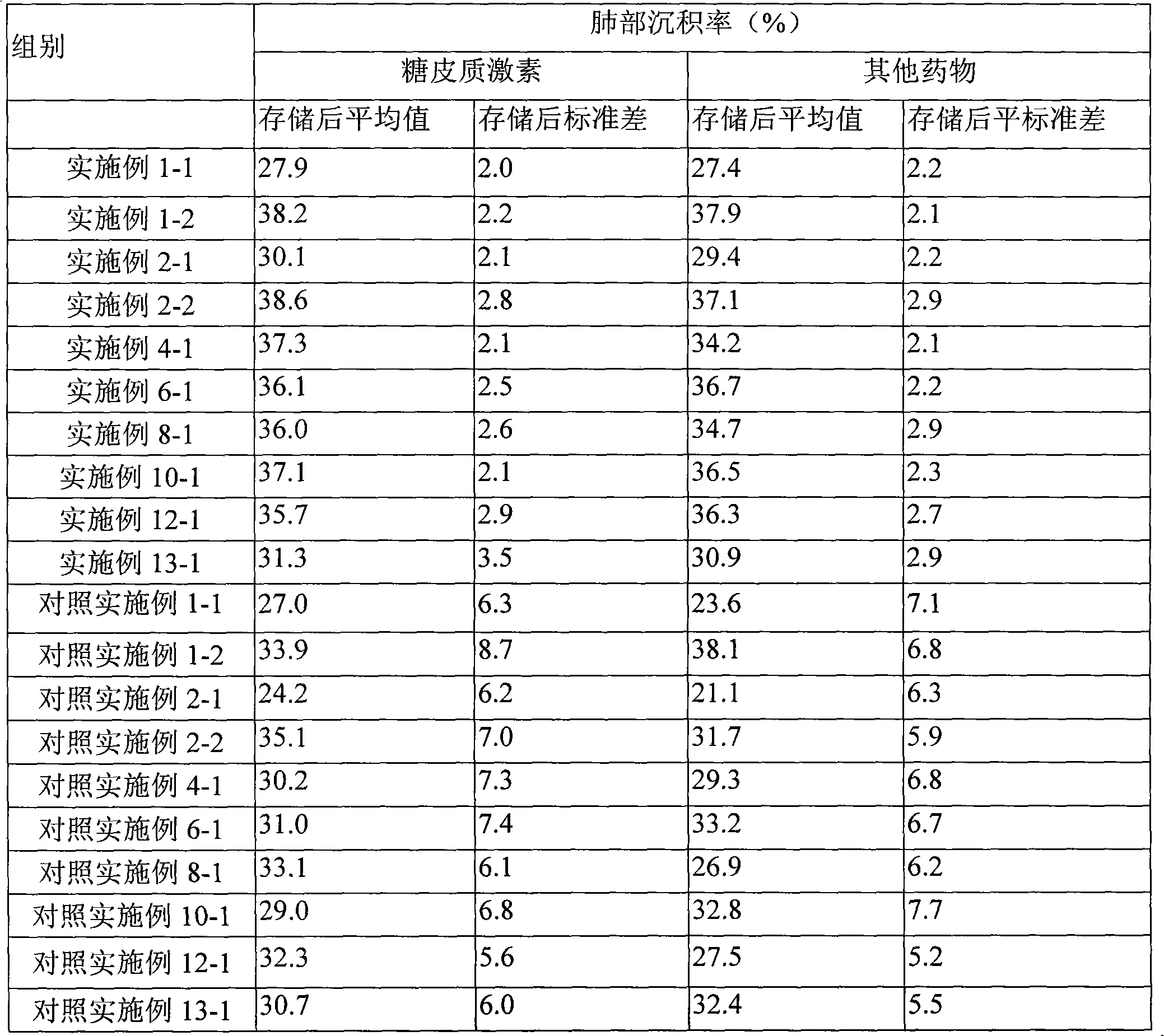
Embodiment 2
[0068] The preparation of comparative example 2 budesonide, formoterol fumarate granules
[0069] Accurately weigh 1 g of budesonide raw material, evenly disperse in 100 ml of deionized water, and stir at high speed for 20 min to make it into a uniform suspension. The above suspension is subjected to high-pressure homogenization through multiple cycles under a certain pressure, and the pipeline is cooled with ethanol at -20°C. The budesonide slurry after high-pressure homogenization was spray-dried to obtain dry powder particles with an average particle size of 3.59 μm, which was set aside. The operating parameters of spray drying are: feed rate 20ml / min, inlet temperature 115°C, additional air 0.55MPa.
[0070] Accurately weigh 0.06g of formoterol fumarate raw material, evenly disperse in 100mL of deionized water, stir at high speed for 10min, and spray dry to obtain dry powder particles with an average particle size of 1.92μm, and set aside. The operating parameters of spr...
Embodiment 3
[0071] The preparation of comparative example 3 mometasone furoate granules
[0072] Accurately weigh 1g of mometasone furoate API, evenly disperse in 100mL of deionized water, stir at high speed for 20min to make it into a uniform suspension. The above suspension is subjected to high-pressure homogenization through multiple cycles under a certain pressure, and the pipeline is cooled with ethanol at -20°C. After high-pressure homogenization, the mometasone furoate slurry was spray-dried to obtain dry powder particles with an average particle size of 2.99 μm, which was set aside. The operating parameters of spray drying are: feed rate 20mL / min, inlet temperature 115°C, additional air 0.55MPa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com