Recombinant HSV (Herpes Simplex Virus) amplicon vector and application thereof
A technology of amplicon and carrier, which is applied in the field of construction of HSV amplicon carrier, can solve the problems of cumbersome operation, low concentration of small circle amplicon DNA, and high cost, and achieve simple construction and preparation, avoid silencing effect, The effect of avoiding pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The construction of embodiment 1, BAC-HSV-1 HF strain
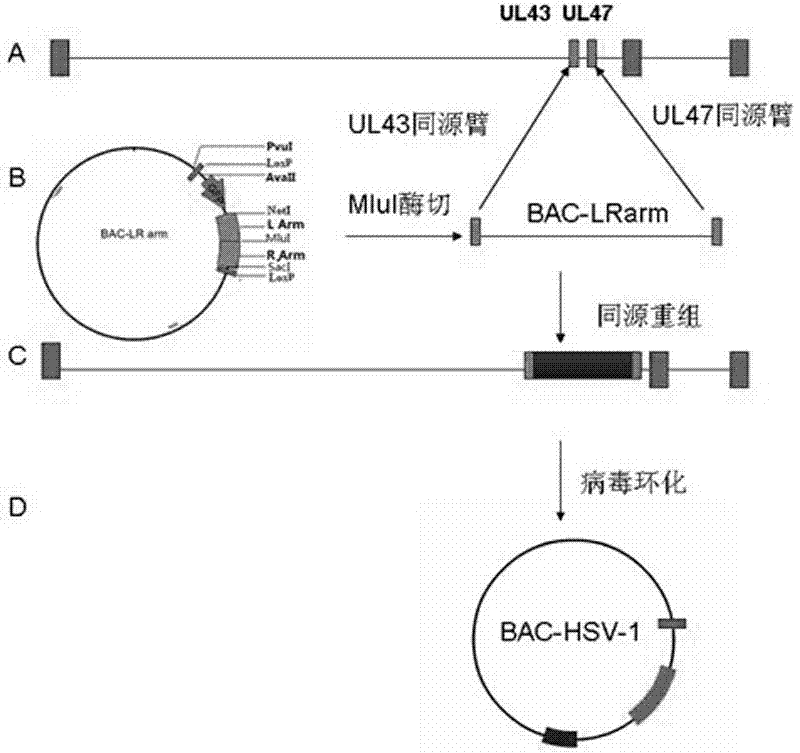
[0062] Such as figure 1 Construction patterns of the indicated BAC-HSV-1 HF strains.
[0063] Using the genome of the HSV-1 HF strain as a template, primers SEQ ID NO: 4 and SEQ ID NO: 5: amplify the upstream homology arm homologous to the UL43 gene of HSV-1, and the two ends of the primers are designed with SacI and MluI enzyme cut linker;
[0064] With primers SEQ ID NO: 6 and SEQ ID NO: 7, amplify the downstream homology arms homologous to the UL47 gene sequence of HSV-1, the enzyme-cut joints of NotI and MluI are designed respectively at the two ends of the primers, and the enzyme-cut ligation method will The homology arm was cloned into the SacI and NotI restriction sites of the BAC plasmid, and the BAC-LR arm was constructed. The BAC containing the HSV-1 homology arm was cut into linear with MluI enzyme, and the linear BAC-HSV-1 homology The source arm and HSV-1 genome were transfected into Vero cells by...
Embodiment 2
[0066] The construction of embodiment 2, HSV-1 amplicon plasmid vector
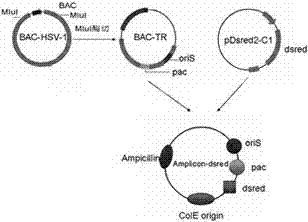
[0067] Such as image 3 HSV-1 amplicon plasmid vector construction model shown.
[0068] 1. Acquisition of "pac" sequence
[0069] Digest BAC-HSV-1HF with MluI enzyme to obtain BAC-TR containing the terminal repeat region; digest BAC-TR with SacI to obtain a 4Kb fragment containing the "pac" sequence of the HSV-1 terminal repeat region; after digesting the 4Kb fragment with HphI A 1.3Kb fragment containing the "pac" sequence of the terminal repeat region was obtained. In addition, the 1.3Kb fragment can be digested with BsrBI to obtain the 188bp Ub-DR1-Uc structure (the packaging signal of the smallest packaging unit). EcoRI digested pGEMT linear T vector (purchased from Dalian Bao Biological Co., Ltd.), Klenow enzyme (purchased from Dalian Bao Biological Co., Ltd.) filled the pGEMT linear T vector after EcoRI digestion, and added the 188bp fragment into pGEMT with a blunt-ended end to T7 univers...
Embodiment 3
[0079] Embodiment 3, construction of recombinant adenovirus Adv-loxP-OPD-loxP and Adv-loxP-D-loxP and virus packaging
[0080] Such as Figure 7 Construction mode of Adv-loxP-OPD-loxP shown.
[0081] 1. Construction of pENTR-loxP-LRarm-loxP vector
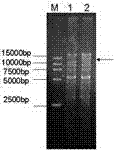
[0082] pENTR-MCS was digested with SalI and BamHI, and the 2.6kb vector pENTR-MCS was recovered from the gel. Cut out the two loxPs in the same direction, the LR homology arm and the fragment of the GFP reporter gene in the middle, and fill in the fragments. The vector and the fragment were connected, transformed, identified as the pENTR-loxP-LRarm-loxP vector, and identified by AvaII digestion. Theoretically, the sizes should be: 3.1Kb, 1.4Kb, 1.2Kb, 440bp, 282bp, and 153bp. The electrophoresis results are shown by the arrows in Figure 8-1A. It can be seen that 3.1Kb, 1.4Kb, 1.2Kb, 440bp, 282bp, and 153bp band. The results showed that the pENTR-loxP-LRarm-loxP vector was constructed successfully.
[0083] 2. Construction o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com