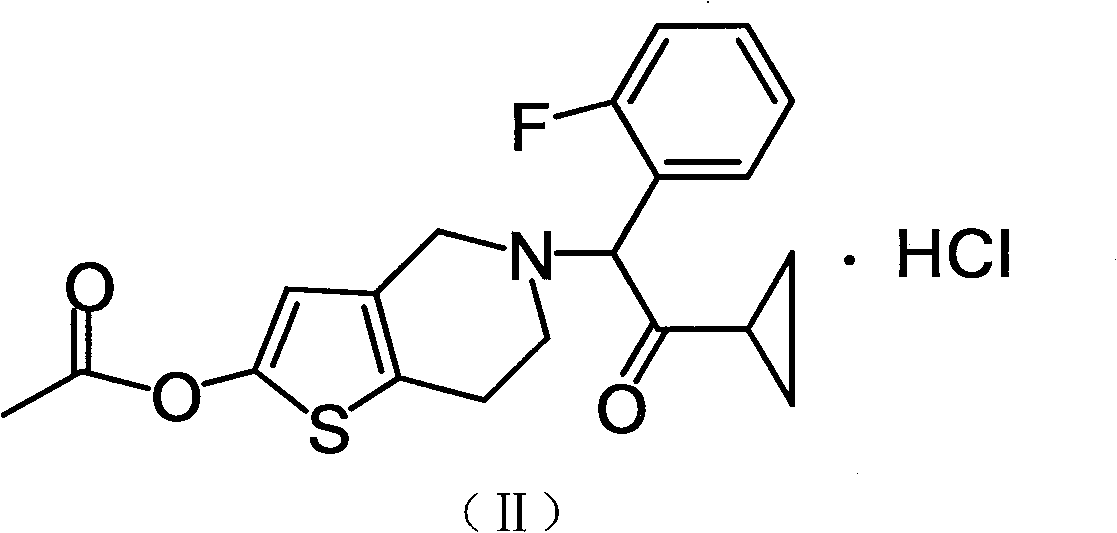
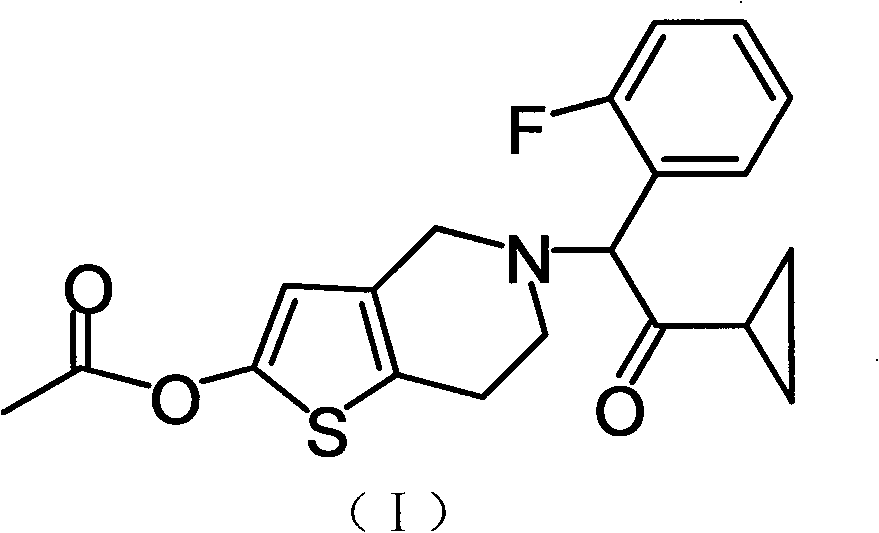
Prasugrel hydrochloride acetic acid solvate as well as crystal and preparation method thereof
A technology of hydrochloride acetic acid and solvates, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problems of poor water solubility, achieve good stability and solubility, and have a simple preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
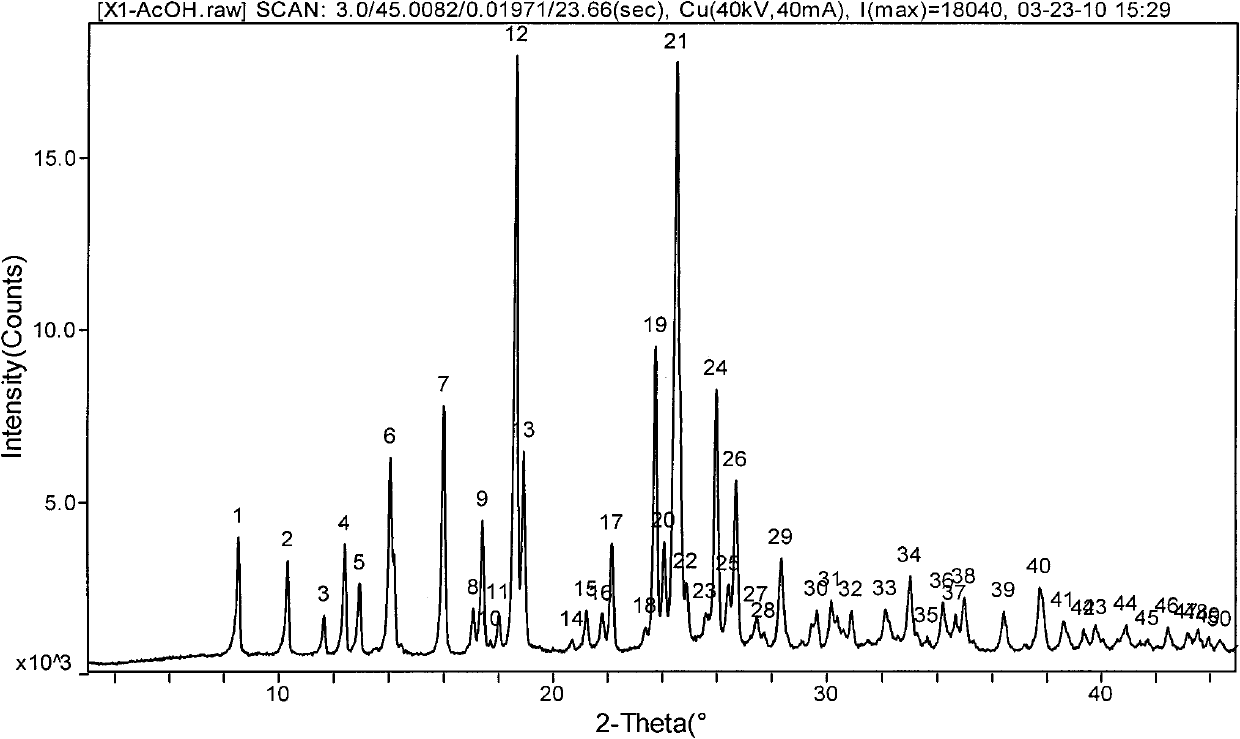
[0025] 2-Acetoxy-5-(α-cyclopropylcarbonyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine hydrochloride acetic acid solvate
[0026] 2-Acetoxy group-5-(alpha-cyclopropylcarbonyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine (compound of formula I) ( 2g) was dissolved in 16ml of acetic acid, and an acetic acid solution containing 1 equivalent of hydrogen chloride was added dropwise with stirring at room temperature, crystals were precipitated in about 10 minutes, and stirred at the same temperature for 2 hours. The precipitated crystals were filtered off, washed with ethyl acetate, and dried under reduced pressure at 60°C for 4 hours to obtain 2.2 g of the title compound as white crystals (yield 87.3%).
Embodiment 2
[0028] 2-Acetoxy-5-(α-cyclopropylcarbonyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine hydrochloride acetic acid solvate
[0029] Dissolve 2-acetoxy-5-(α-cyclopropylcarbonyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine (2g) in 8ml In a mixed solvent of acetic acid and 8 ml of ethyl acetate, an ethyl acetate solution containing 1 equivalent of hydrogen chloride was added dropwise with stirring at room temperature, a small amount of seed crystals (crystals obtained in Example 1) were added, and stirred at the same temperature for 2 hours. The precipitated crystals were filtered off, washed with ethyl acetate, and dried under reduced pressure at 60°C for 4 hours to obtain 2.3 g of the title compound as white crystals (91.3% yield).
Embodiment 3
[0031] 2-Acetoxy-5-(α-cyclopropylcarbonyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine hydrochloride acetic acid solvate
[0032] Dissolve 2-acetoxy-5-(α-cyclopropylcarbonyl-2-fluorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine (2g) in 16ml In acetic acid, an aqueous solution containing 1 equivalent of hydrogen chloride was added dropwise with stirring at room temperature, a small amount of seed crystals (crystals obtained in Example 1) were added, and the mixture was stirred at the same temperature for 2 hours. The precipitated crystals were filtered, washed with ethyl acetate, and dried under reduced pressure at 60°C for 4 hours to obtain 2.2 g of the title compound as white crystals (yield 87.3%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com