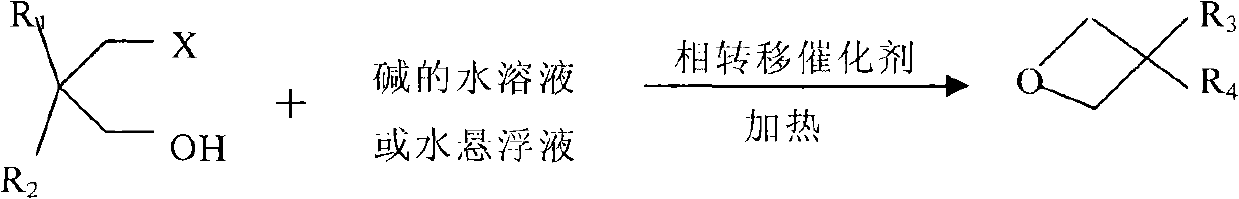
Synthesis method for oxetane compounds
A technology for oxetane and a synthesis method, applied in directions such as organic chemistry, can solve the problems of complicated separation and purification of products and organic solvents, unfavorable industrial production of oxetane, large environmental pollution, etc., and achieves high utilization rate , less by-products, less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 3-ethyl-3-bromomethyl oxetane
[0034] With 37.75 gram (0.125mol) 2,2-bisbromomethyl-1-butanol acetate, 0.402 gram (0.00125mol) tetrabutylammonium bromide, 48.0 gram mass fraction 25% sodium hydroxide (0.3mol ) solution was added to the reaction flask together. Start to stir, heat to keep the reaction temperature at 70°C for 3 hours, and the reaction ends. 21.87 g of the organic phase was separated, and the content of the product 3-ethyl-3-bromomethyloxetane in the organic phase was analyzed by gas chromatography to be 76.0%. The reaction yield reached 74.3%.
Embodiment 2
[0035] Example 2 Preparation of 3-hydroxymethyl-3-bromomethyloxetane
[0036] Add 65.5 g (0.25 mol) of dibromoneopentyl glycol, 1.61 g (0.005 mol) of tetrabutylammonium bromide, and 60.0 g of 20% sodium hydroxide (0.3 mol) solution into the reaction flask. Start to stir, heat to keep the reaction temperature at 30°C for 6 hours, continue to heat up to 75°C for 1.5 hours, and the reaction ends. 35.75 g of the organic phase were separated off. With 15.0 grams of dichloromethane, extract the aqueous phase twice, and combine the organic phases to obtain 59.85 grams of organic phases. The content of product 3-hydroxymethyl-3-bromomethyloxetane in the gas chromatography analysis organic phase is 65.78%. The reaction yield reached 87%.
Embodiment 3
[0037] Embodiment 3 3, the preparation of 3-bismethyloxetane
[0038] 41.75 grams (0.25mol) of 2-methyl-2-bromomethylpropanol, 3.24 grams (0.0125mol) of tetrabutylammonium hydroxide, and 44.45 grams of sodium hydroxide (0.5mol) solution with a mass fraction of 45% were added together for reaction in the bottle. Stirring was started, and the reaction temperature was maintained at 40° C. for 10 hours by heating. After the reaction was completed, 20.65 grams of the organic phase was separated, and the content of the product 3 in the organic phase was analyzed by gas chromatography, and the content of 3-bismethyloxetane was 76.0%. The reaction yield reached 73.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com