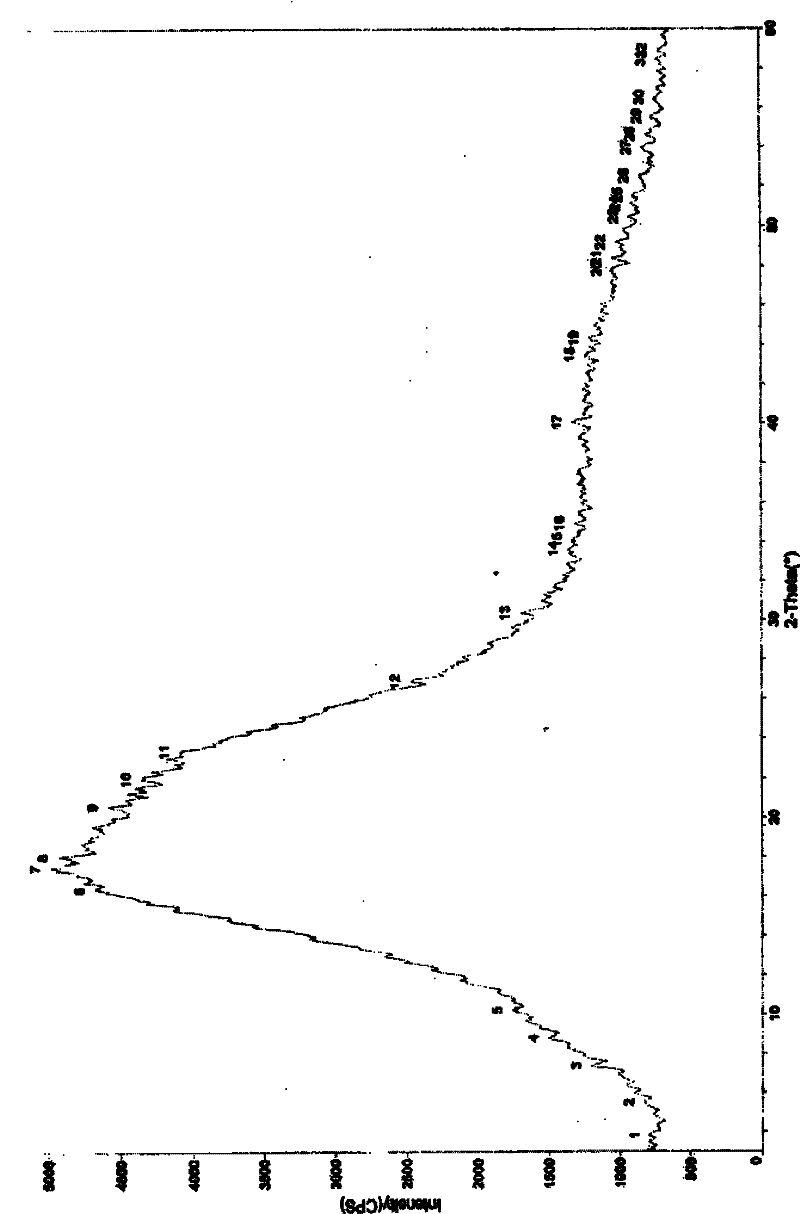
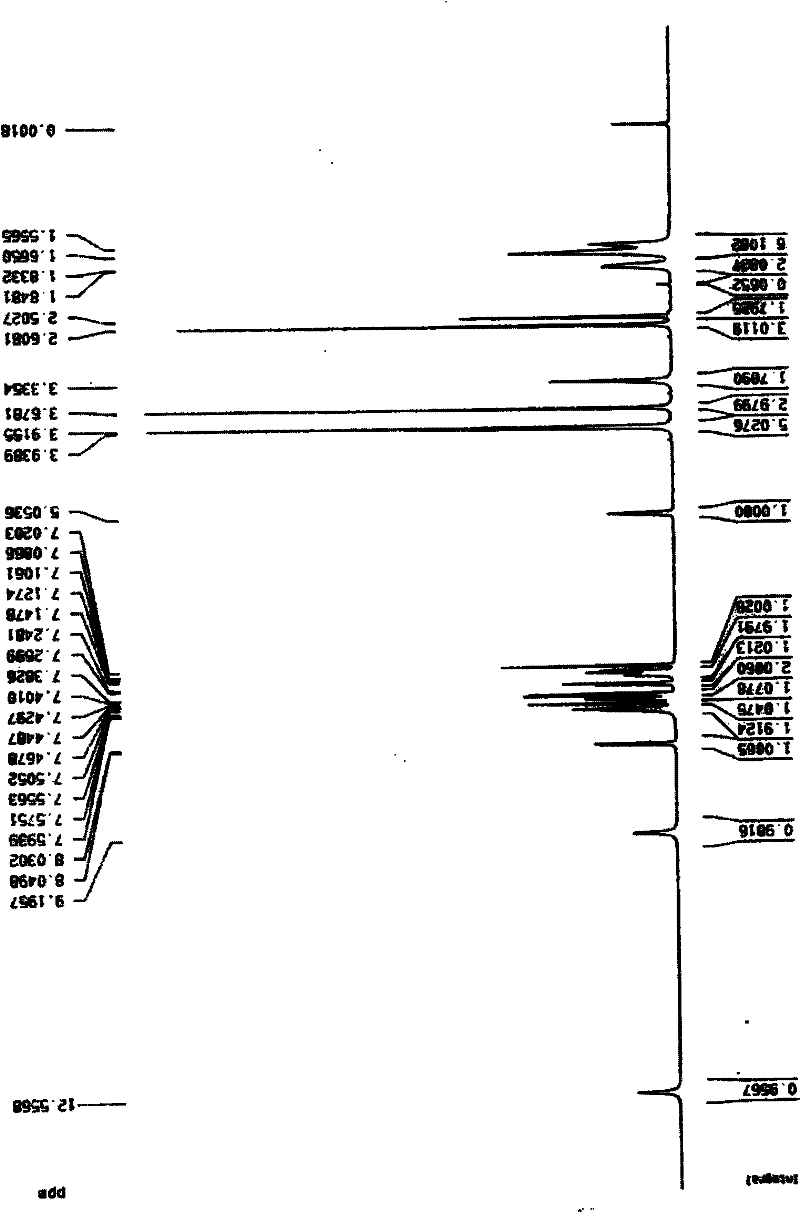
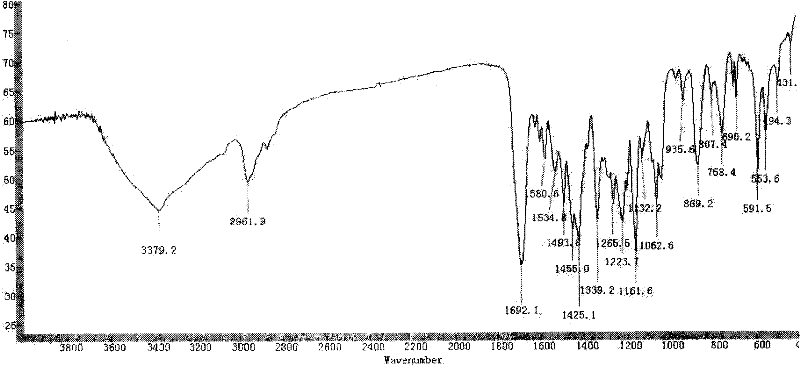
Method for preparing amorphous zafirlukast solid
An amorphous, solid technology, applied in the direction of organic chemistry, can solve the problems of poor yield, complex process, high conditions, achieve good dissolution properties, simplify the production process, and avoid the effects of micronization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of amorphous zafirlukast method 1
[0025] Put 10g of zafirlukast in a 250ml round-bottomed flask, add 50ml of acetone, stir at room temperature until fully dissolved, and then vacuumize to 2.65×10 on a rotary evaporator with a water pump 3 Pa. Soak the flask in a water bath preheated to 40°C, evaporate to complete dryness, and scrape off the product after cooling to obtain amorphous zafirlukast in the form of white powder.
Embodiment 2
[0026] Example 2 Preparation of amorphous zafirlukast method 2
[0027] Put 10g of zafirlukast in a 250ml round-bottomed flask, add 50ml of acetonitrile, stir at room temperature until completely dissolved, and then vacuumize to 2.65×10 on a rotary evaporator with a water pump. 3 Pa, immerse the flask in a water bath preheated to 40°C, evaporate to complete dryness, and scrape off the product after cooling to obtain amorphous zafirlukast in the form of white powder.
Embodiment 3
[0028] Example 3 Preparation of amorphous zafirlukast method 3
[0029] Put 10g of zafirlukast in a 250ml round-bottomed flask, add 50ml of 1,4-dioxane, stir at room temperature until fully dissolved, and then pump vacuum to 2.65×10 on the rotary evaporator 3 Pa, immerse the flask in a water bath preheated to 30°C, evaporate to complete dryness, heat up to 60°C for 1 hour, and use an oil pump to evacuate to 1×10 2 Pa, heated to 80°C for 1 hour. The product was scraped off after cooling to obtain amorphous zafirlukast as a white powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com