Tetracaine hydrochloride multi-vesicular liposome freeze-dried powder and preparation method thereof
A technology of tetracaine hydrochloride and multivesicular liposome, which is applied in the field of sustained-release preparations of tetracaine hydrochloride, and can solve problems such as leakage of encapsulated substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Precisely weigh 60 mg of hydrogenated soybean lecithin, 30 mg of cholesterol, and 1 mg of glyceryl trilaurate and dissolve them in 2.4 ml of chloroform as the lipid phase;
[0027] 2. Preparation of Rosa Rosa extract: add 500 mg of water to 50 mg of washed Rosa Rosa fruit, ultrasonically break for 1 min, the ultrasonic frequency is 20 kHz, and centrifuge for 3 min at 10,000 rpm, take the supernatant to obtain Rosa Rosa Extraction solution;
[0028] 3. Precisely weigh 80 mg of tetracaine hydrochloride and dissolve it in water, add the Rosa rose fruit extract obtained in step 2 as the inner water phase, so that the final concentration of tetracaine hydrochloride is 40 mg / ml;
[0029] 4. Precisely weigh 99mg of glucose and dissolve it in 11ml of water, as the external water phase, the concentration of glucose is 9mg / ml;
[0030] 5. Slowly add 2ml of the inner water phase to the upper layer of 2.4ml of the lipid phase, and use a high-speed shear homogenizer to act on it...
Embodiment 2
[0036] 1. Precisely weigh 60 mg of hydrogenated soybean lecithin, 30 mg of cholesterol, and 1 mg of glyceryl trilaurate and dissolve them in 2.4 ml of chloroform as the lipid phase;
[0037] 2. Precisely weigh 80 mg of tetracaine hydrochloride and dissolve it in water as the inner water phase, so that the final concentration of tetracaine hydrochloride is 40 mg / ml;
[0038] 3. Precisely weigh 99mg of glucose and dissolve it in 11ml of water, as the external water phase, the concentration of glucose is 9mg / ml;
[0039] 4. Slowly add 2ml of the inner water phase to the upper layer of 2.4ml of the lipid phase, and use a high-speed shear homogenizer to act on it at a speed of 10,000rpm for 10min to obtain a water-in-oil emulsion;
[0040] 5. Add the above-mentioned emulsion to 11ml of the external water phase, and use a high-speed shear homogenizer to act on it at a speed of 10,000rpm for 10min to obtain a water-in-oil-in-water type double emulsion;
[0041] 6. Add the double emu...
Embodiment 3
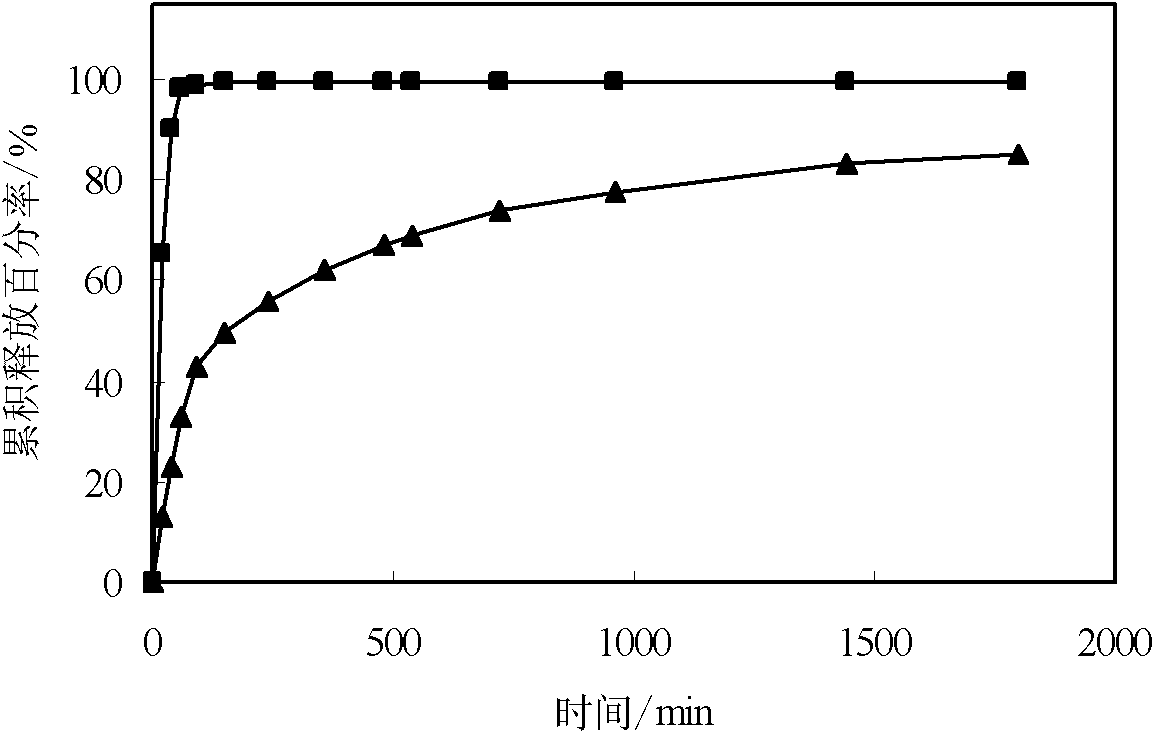
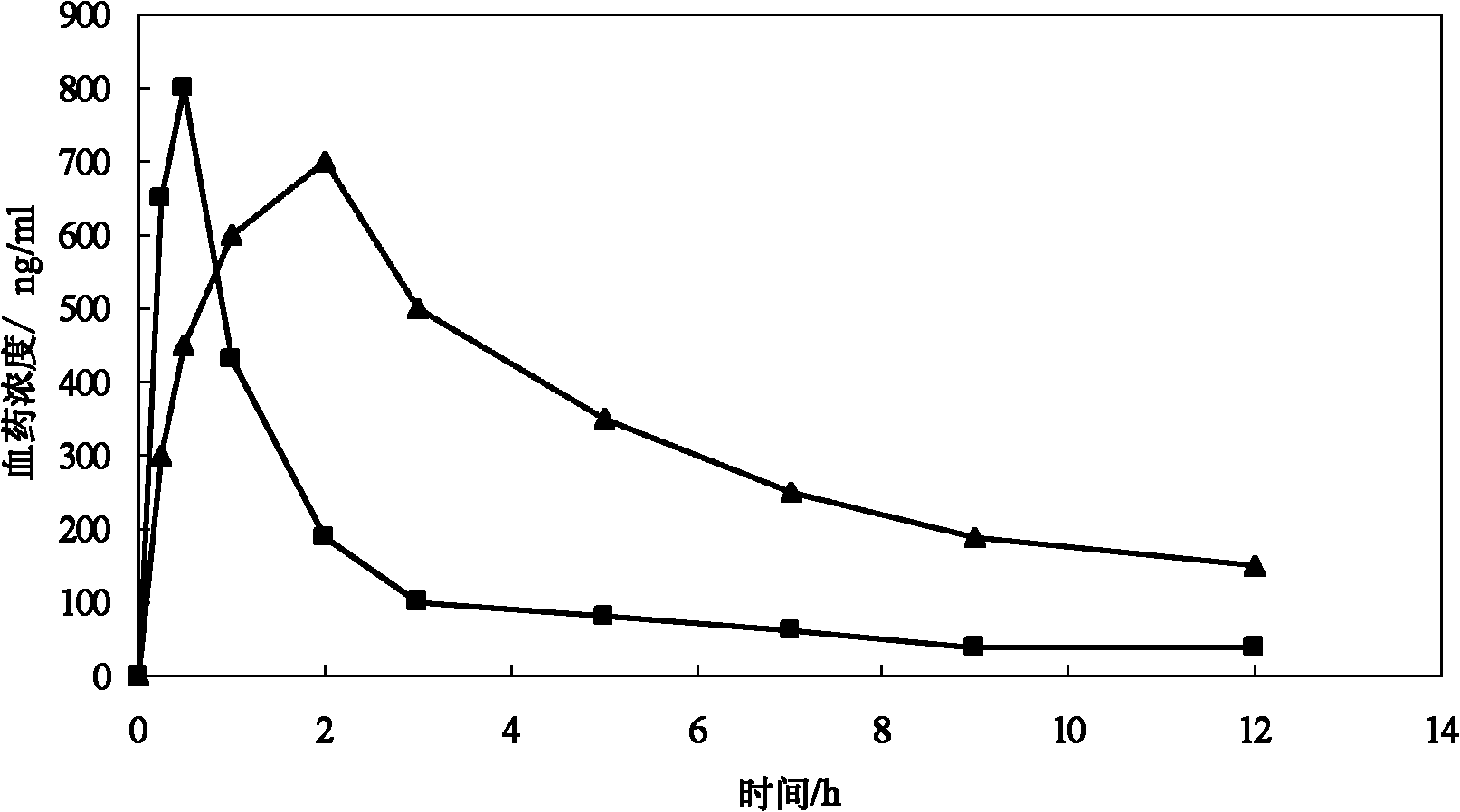
[0045] The tetracaine hydrochloride multivesicular liposome freeze-dried powder of experimental group I is dissolved in water, and when the tetracaine hydrochloride in the liposome is all released, the concentration of tetracaine hydrochloride in the solution is 7.2mg / ml 5ml of the above-mentioned solution is taken and placed in a dialysis bag, and the corresponding concentration of tetracaine hydrochloride aqueous solution is prepared to be 7.2mg / ml, and this solution of 5ml is placed in a dialysis bag, and released simultaneously in timed release in dissolution cups with 250ml distilled water respectively, The rotation speed is 100 rpm, and the temperature is 37°C. Take samples at 20min, 40min, 60min, 90min, 150min, 240min, 360min, 480min, 540min, 720min, 960min, 1440min, 1800min respectively, and measure its concentration with HPLC (Chinese Pharmaceutical Industry Impurities, 2010, 41 (6), 453-455 ), draw the drug-time curve of tetracaine hydrochloride polyvesicular liposom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com