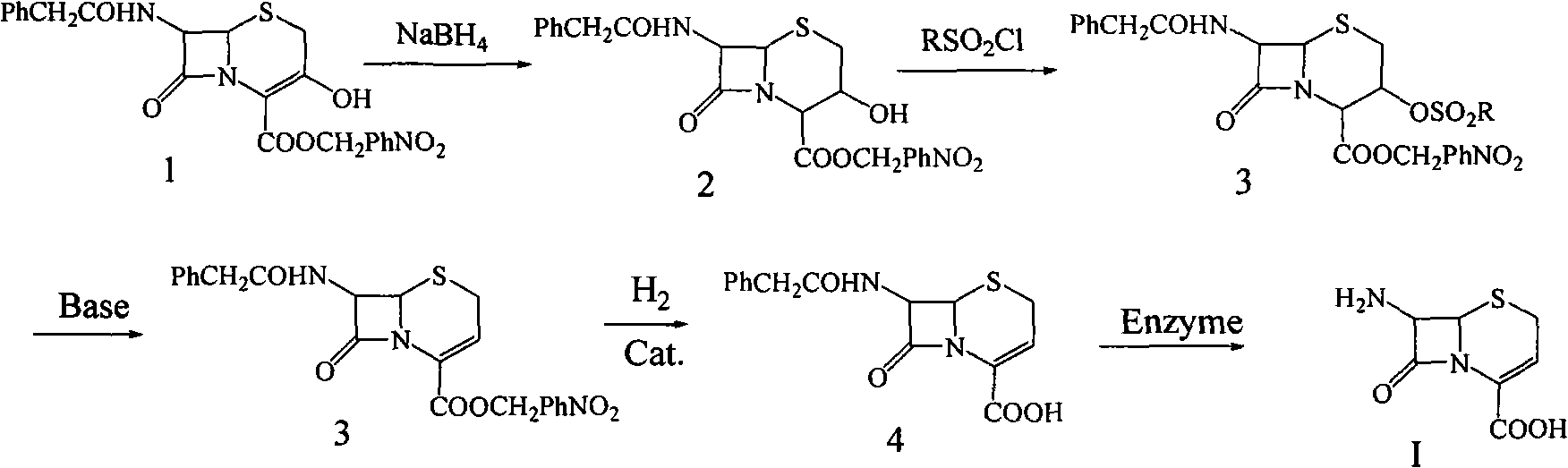
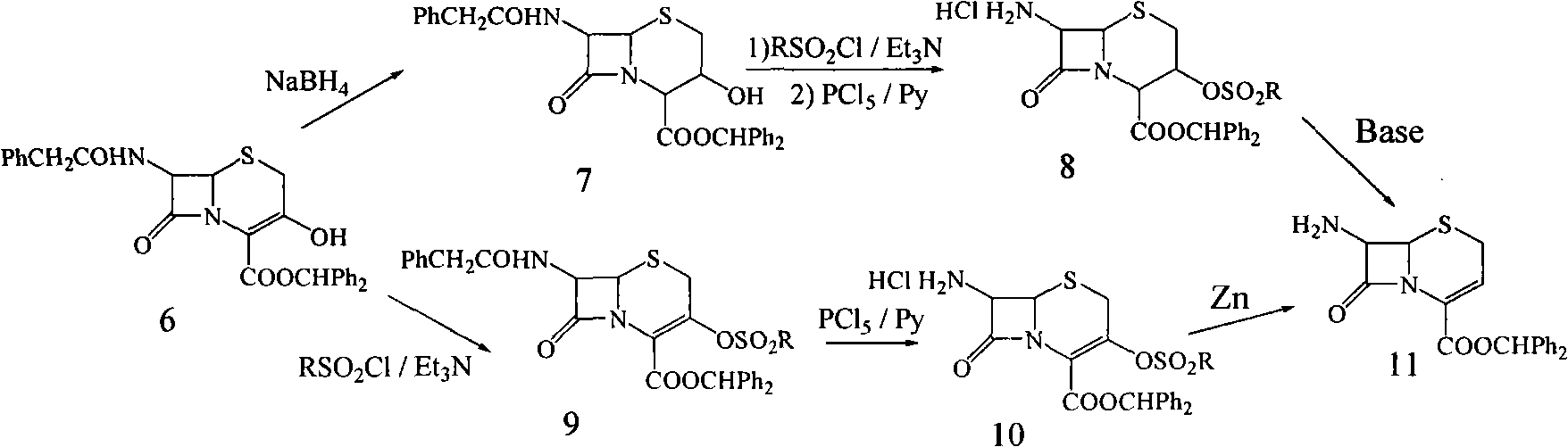
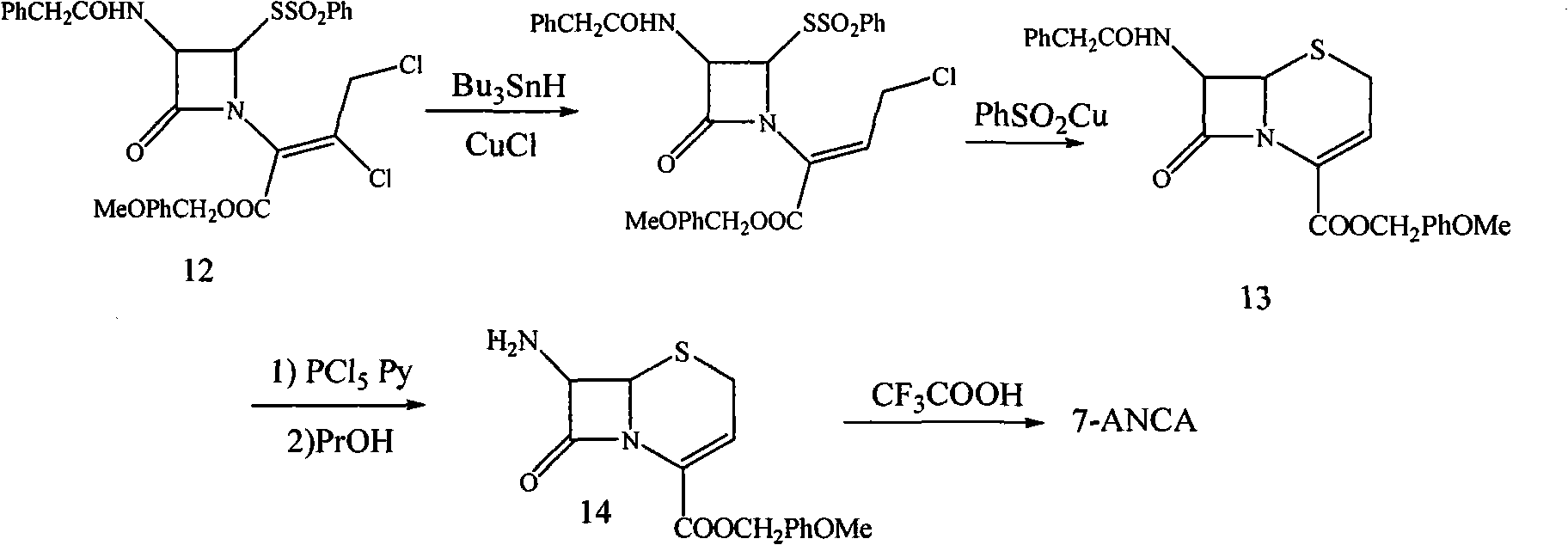
Preparation method for 7-amino-3-non-3-cephem-4-carboxylic acid(7-ANCA)
A technology of 7-ANCA and hydroxycephalosporanic acid, which is applied in the field of medicine and chemical industry, can solve the problems of large equipment investment, harsh reaction conditions, and high raw material consumption, and achieve the effects of less equipment investment, mild reaction conditions, and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] (1), the synthesis of 7-phenylacetamido-3-cephem-4-carboxylic acid diphenylmethyl ester (IV-1)
[0036] Add 50.2g (0.1mol) of compound II-1 and 600mL of toluene into a 1000mL reaction flask, stir for 20min, and cool down to -5~0°C; add 13.74g (0.12mol) of methanesulfonyl chloride. At 0°C, 13.13 g (0.13 mol) of triethylamine was added dropwise within 1-1.5 h, and stirring was continued for 0.5 h after the addition was complete. Within 1 h, 10.95 g (0.15 mol) of diethylamine was added dropwise, and after the addition was complete, stirring was continued for 0.5 h. Add 200 mL of 3% sulfuric acid solution, stir at room temperature for 0.5 h; separate layers, wash the organic layer with 100 mL of 5% sodium bicarbonate solution and 100 mL of water, and concentrate under reduced pressure; add 200 mL of methanol to the residue, stir well, and disperse the precipitated solid. Add 20mL of water, cool down to 0°C, stir slowly for 1h, filter, and dry under reduced pre...
Embodiment 2
[0042]
[0043] (1), the synthesis of 7-phenylacetamido-3-cephem-4-carboxylic acid (V-1)
[0044] Add 80mL of dichloromethane into a 500mL dry reaction flask, cool down to 0°C, add 20g (0.15mol) of aluminum trichloride, and stir for 30min; at 5°C, add dropwise compound IV-136.3g (0.075mol), A mixed solution of 16.2 g (0.15 mol) of methyl ether in 200 mL of dichloromethane. The addition was completed within 3h, and the reaction was continued to stir for 1h at room temperature. The temperature was lowered below 0°C, and the reaction solution was slowly added to 300 mL of 3% hydrochloric acid solution, and stirred at room temperature for 1 h after the addition was complete. After filtration, the solid was washed with 100 mL of cold water, and dried under reduced pressure at 40° C. to constant weight to obtain 21.9 g of a light pink solid with a yield of 91.8%. After the filtrate was layered and dried, dichloromethane was recovered.
[0045] (2) Synthesis of 7-phenylacetamid...
Embodiment 3
[0048]
[0049] (1) Synthesis of 7-amino-3-non-3-cephem-4-carboxylic acid (7-ANCA)
[0050] Suspend 31.8g (0.1moL) of compound V in 500mL of water, stir for 10min, add dropwise 100mL of 1% sodium hydroxide solution, add 3g of activated carbon, and stir at room temperature for 1h; filter out the activated carbon, wash with 20mL of water, and combine with the filtrate. Add 30 g of penicillin hydrolase, stir for 10 min, and add 12.6 g (0.15 mol) of sodium bicarbonate solid in 3 times within 2 h. The reaction was stirred at 30-33°C and the progress of the reaction was monitored by TLC. After the reaction, the enzyme was filtered out, washed with 80ml of water, and combined with the filtrate; 2g of activated carbon was added, stirred for 0.5h to decolorize; the activated carbon was filtered out. At room temperature, adjust the pH to 6 with 15% hydrochloric acid, stir for 0.5 h; cool down to below 5°C, continue to adjust the pH to 5 with 10% hydrochloric acid, stir slowly for 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com