Biodegradable polymer, preparation method thereof and nucleic acid drug delivery carrier
A polymer and synthesis method technology, applied in the field of life medicine, can solve the problems of toxicity, immune stimulation, and low transfection efficiency, and achieve good stability, high reproducibility, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049]3. Preparation and Stability Test of Compound Nanoparticles of Formula I
[0050] The amphiphilic copolymer can be prepared into nanoparticles in aqueous solution by conventional methods, such as solvent evaporation and dialysis.
[0051] Solvent evaporation method: Dissolve the copolymer in tetrahydrofuran, add ultrapure water dropwise under stirring to obtain the corresponding final concentration, after stirring for two hours, remove the organic solvent under reduced pressure, and then set the volume to a suitable volume.
[0052] Dialysis method: Dissolve the copolymer in a good solvent such as dimethyl sulfoxide (DMSO), and drop into ultrapure water under stirring to obtain the corresponding final concentration. After stirring for two hours, dialyze in a dialysis bag to remove the organic solvent.
[0053] The nanoparticles of each carrier material provided by the invention are prepared by a solvent volatilization method, and the particle size distribution and surfac...
Embodiment 1
[0068] Embodiment 1, the synthesis of single / double hydroxyl polyhydroxyalkanoate and its end group modification
[0069] a. Synthesis and characterization of mono / dihydroxy polyhydroxyalkanoate (mPHA-OH / PHA-diol)
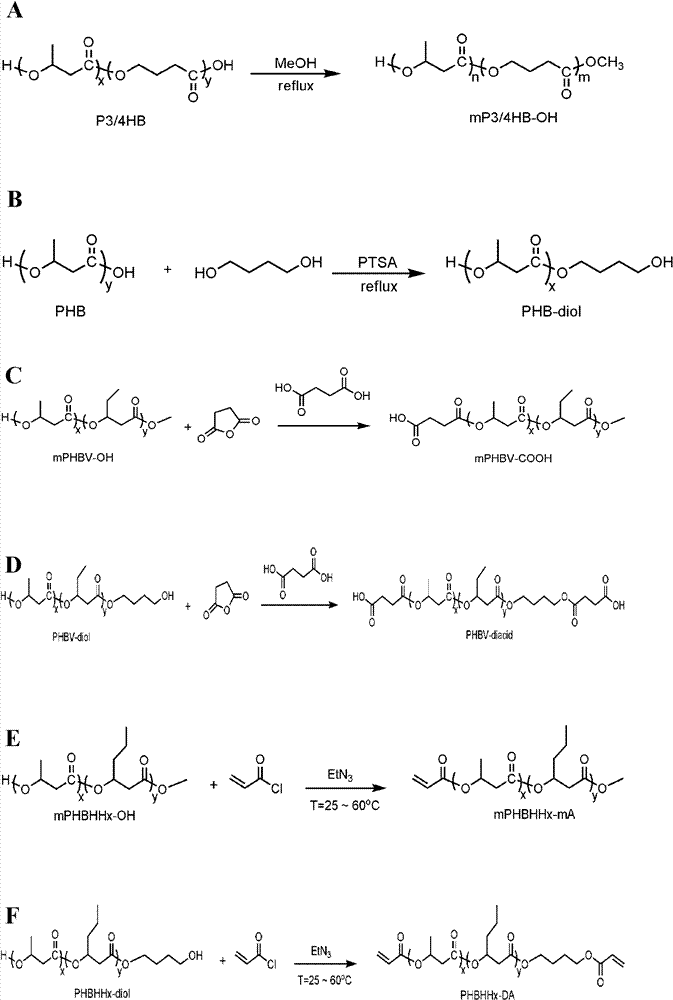
[0070] The synthesis of various mono / dihydroxy polyhydroxyalkanoate (mPHA-OH / PHA-diol) is prepared by transesterification reaction with methanol or diol under the catalysis of p-toluenesulfonic acid (PTSA). The alcohol may be ethylene glycol, 1,3-propanediol, 1,4-butanediol, 1,6-hexanediol. All kinds of polyhydroxyalkanoate raw materials in the present invention are purchased from Shenzhen Ecoman Biotechnology Co., Ltd. The operation steps of this type of reaction are illustrated below by taking monomethyl poly-3-hydroxybutyrate-4-hydroxybutyrate (mP3 / 4HB-OH) and polyhydroxybutyrate diol (PHB-diol) as examples.
[0071] The synthetic route of mP3 / 4HB-OH is as follows figure 1 (A) shown. mP3 / 4HB-OH is prepared by transesterification of P3 / 4HB and methanol under ...
Embodiment 2
[0081] Embodiment 2, synthesis and characterization of polyhydroxyalkanoate and cationic compound copolymer
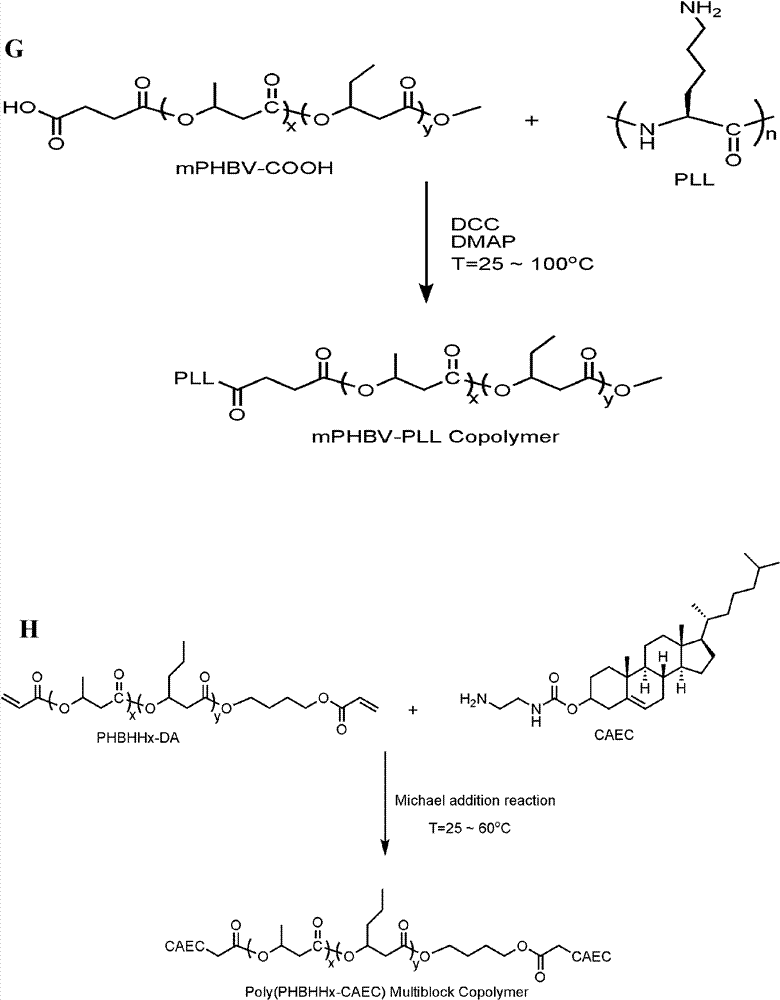
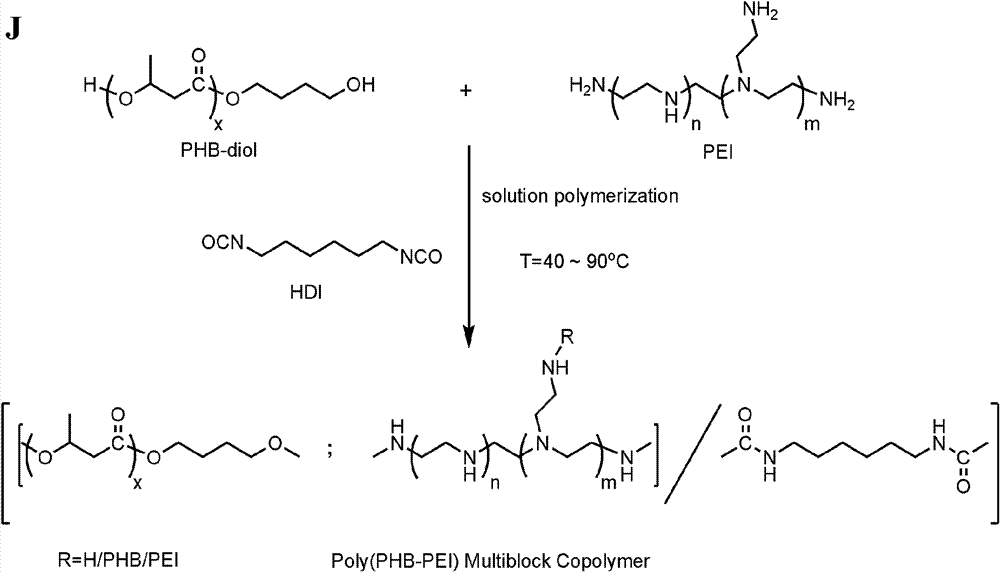
[0082] Copolymers of polyhydroxyalkanoates of different types and molecular weights with cationic peptides, cationic lipids, and cationic polymers are based on mono / dihydroxy, mono / dicarboxy, mono / dipropenyl groups of corresponding types and molecular weights. Polyhydroxyalkanoates and cationic peptides, cationic esters, and cationic polymers, with HDI / CDI / IPDI / DCC / EDC as linkers or condensation agents, in stannous octoate (Sn(Oct) 2 ) or 4-dimethylaminopyridine (DMAP) under the catalysis of solution polymerization, condensation or Michael addition and other methods; by adjusting the molecular weight of polyhydroxyalkanoate and cationic compound and the ratio of the two can get different molecular weight , Copolymers of different compositions. Due to its high catalytic activity and non-toxicity, stannous octoate is the most widely used polymerization catalyst and has ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com