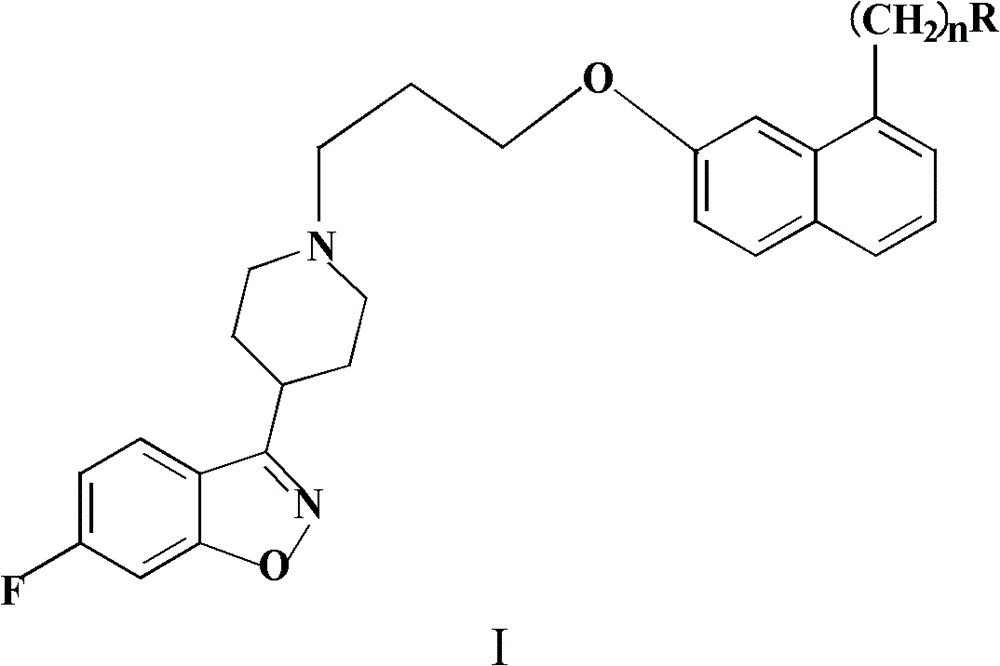
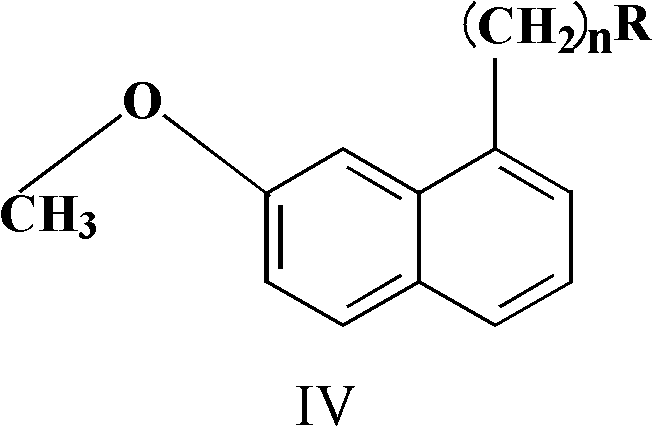
N-(naphthoxyalkyl)heteroarylpiperidine compounds and preparation method and applications thereof
A compound, piperidine technology, applied in the field of drug synthesis, can solve the problem of single action site of antipsychotic drugs, and achieve the effect of easy-to-obtain raw materials, mild reaction conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: N-(2-(7-(3-(4-(6-fluorobenzisoxazol-3-yl)piperidin-1-yl)propoxy)naphthalene-1-yl)ethyl base) the preparation of acetamide
[0046] 1. Preparation of N-(2-(7-(3-chloropropoxy)naphthalene-1-yl)ethyl)acetamide
[0047] Add N-(2-(7-methoxynaphthalen-1-yl)ethyl)acetamide 6g (24.6mmol) and 24ml (6N) concentrated hydrochloric acid at room temperature and heat to reflux at 100°C for 9h. After the reaction was detected by TLC (acetone:n-hexane=1:1), cooling and crystallization was carried out. The solid was obtained by filtration and chromatographed on a silica gel column (eluent: acetone:n-hexane=1:1) to obtain 1.4 g of white N-(2-(7-hydroxynaphthalen-1-yl)ethyl)acetamide solid.
[0048] At room temperature, in a 50ml three-necked flask, add 1.4g (6.1mmol) N-(2-(7-hydroxynaphthalene-1-yl)ethyl)acetamide, 2.5g (18.3mmol) of potassium carbonate, 1.9g (12.2mmol) 1-bromo-3-chloropropane and acetone 15ml, reflux at 60°C, stir for 6h, TLC (acetone:n-hexane=1:1) detects ...
Embodiment 2
[0051] Example 2: Acetic acid-O-(2-(7-(3-(4-(6-fluorobenzisoxazol-3-yl)piperidin-1-yl)propoxy)naphthalene-1-yl ) Preparation of ethyl) ethyl ester
[0052] 1. Preparation of acetic acid-2-(7-(3-chloropropoxy)naphthalene-1-yl)ethyl ester
[0053] Add 6 g (24.7 mmol) of acetic acid-2-(7-methoxynaphthalen-1-yl) acetate and 24 ml (6N) concentrated hydrochloric acid at room temperature and heat to reflux at 100° C. for 9 h. After the reaction was detected by TLC (acetone:n-hexane=1:1), cooling and crystallization was carried out. The obtained solid was filtered and chromatographed on a silica gel column (eluent: acetone:n-hexane=1:1) to obtain 1.3 g of white 2-(7-hydroxynaphthalen-1-yl)ethyl)ethyl acetate as a solid.
[0054] At room temperature, in a 50ml three-necked flask, 1.3g (5.7mmol) acetic acid-2-(7-hydroxynaphthalene-1-yl) ethyl) ethyl ester, 2.4g (17.1mmol) of potassium carbonate, 1.8g ( 11.4mmol) 1-bromo-3-chloropropane and 15ml acetone, reflux at 60°C and stir for 6h...
Embodiment 3
[0057] Example 3S-(2-(7-(3-(4-(6-fluorobenzisoxazol-3-yl)piperidin-1-yl)propoxy)naphthalene-1-yl)ethyl) Preparation of thiol acetate
[0058] 1. Preparation of S-(2-(7-(3-chloropropoxy)naphthalene-1-yl)ethyl)thiol acetate
[0059] Add S-(2-(7-methoxynaphthalen-1-yl)ethyl)thiol acetate 6g (23.1mmol) and 24ml (6N) concentrated hydrochloric acid at room temperature and heat to reflux at 100°C for 9h. After the reaction was detected by TLC (acetone:n-hexane=1:1), cooling and crystallization was carried out. The solid was obtained by filtration and passed through a silica gel column (eluent: acetone:n-hexane=1:1) to obtain 1.4 g of white S-(2-(7-hydroxynaphthalen-1-yl)ethyl)thiol acetate solid.
[0060] At room temperature, in a 50ml three-necked flask, add 1.4g (5.7mmol) S-(2-(7-hydroxynaphthalene-1-yl) ethyl) mercaptan acetate, potassium carbonate 2.4g (17.1mmol) successively , 1.8g (11.4mmol) of 1-bromo-3-chloropropane and 15ml of acetone, reflux at 60°C and stir for 6h. Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com