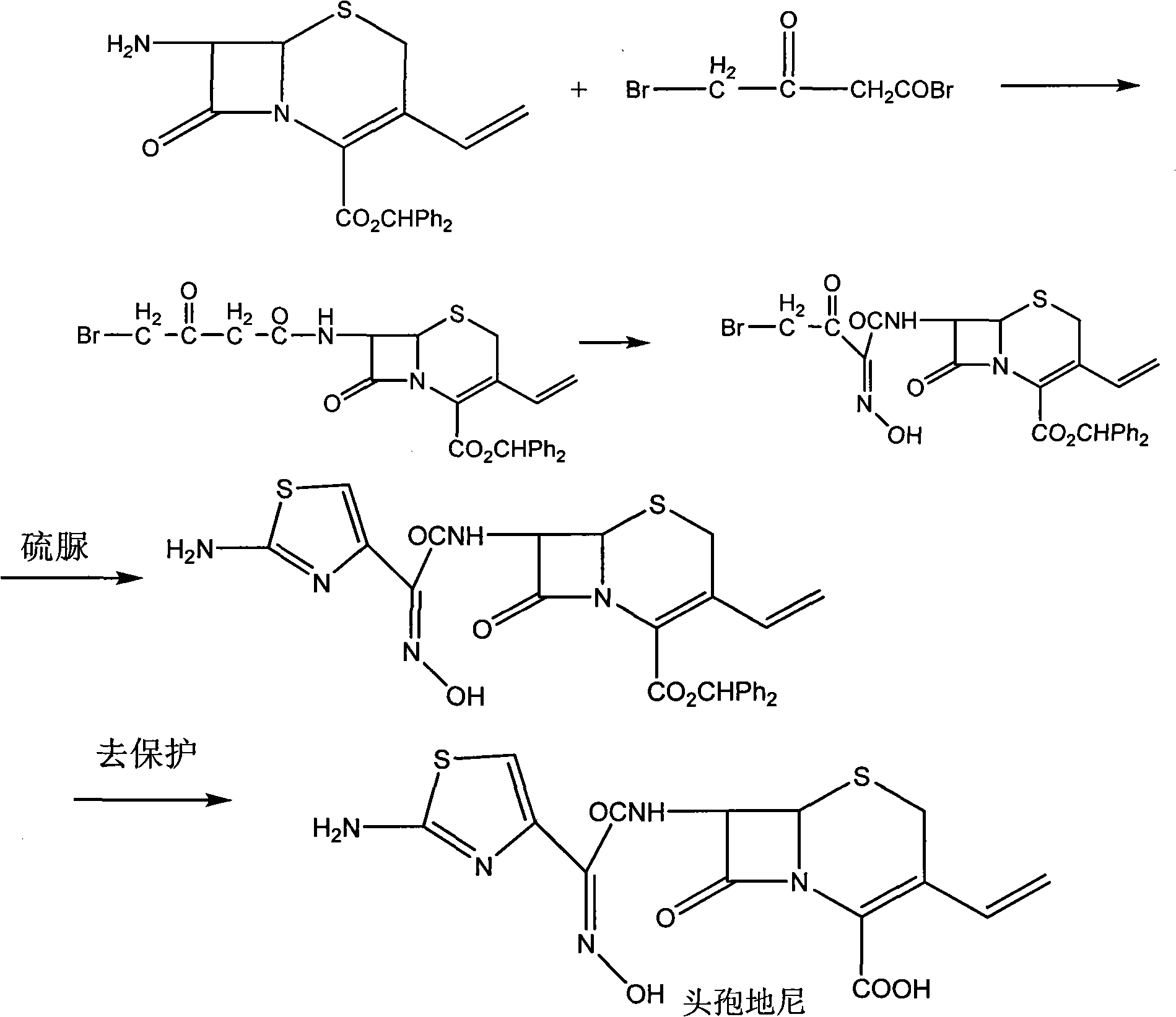
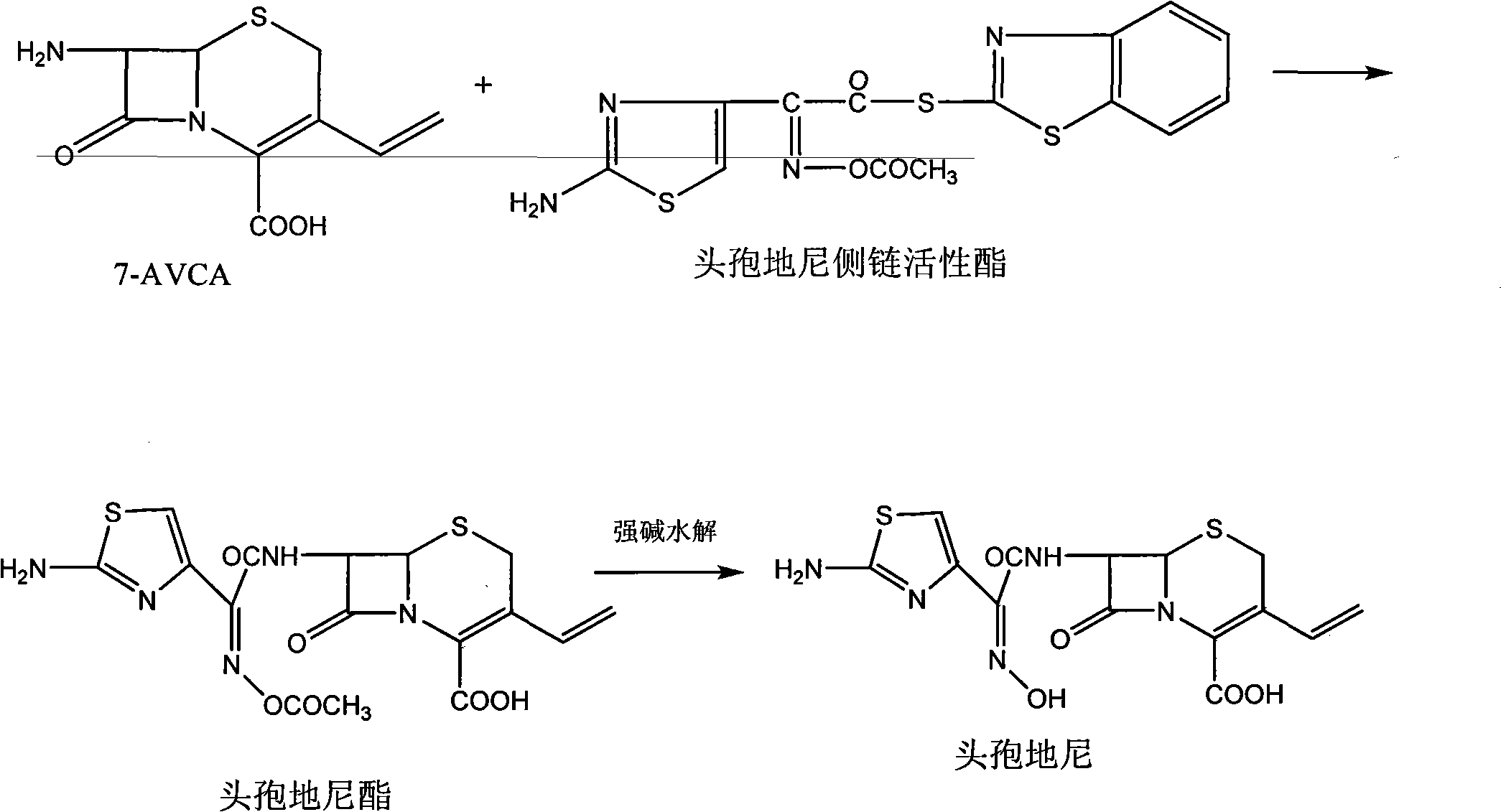
Method for preparing cefdinir
A technology for cefdinir and cefdinir active ester, which is applied in the field of preparation of cefdinir, can solve problems such as difficulty in obtaining raw materials, reduce product yield, prolong production cycle, etc., and achieve shortening production cycle, improving yield and saving The effect of the solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 180mL of purified water, 20.0g of 7-AVCA and 36g of cefdinir active ester (acetoxyimide) into a 500mL four-necked reaction flask, add 140mL of tetrahydrofuran under stirring, cool down to 0°C, add 16mL of triethylamine, and heat up to 20°C, stirred and reacted for 9 hours, sampled and detected by HPLC that the residual amount of 7-AVCA was less than 1.0%, then added 120mL dichloromethane to the reaction solution, stirred for 30 minutes and then stood still for 30 minutes, separated into layers, and the lower dichloromethane phase was reused Extract with 50mL of purified water, stir for 30 minutes, let stand for 30 minutes, separate layers, recover the organic solvent from the lower dichloromethane phase, combine the upper water phase and add 80mL of dichloromethane to wash, stir for 30 minutes, let stand for 30 minutes, and separate layers , the lower dichloromethane phase is used for organic solvent recovery, 2g of activated carbon is added to the upper water phase,...
Embodiment 2
[0035] Add 180mL of purified water, 20.0g7-AVCA and 36g of cefdinir active ester (acetoxyimide) into a 500mL four-necked reaction flask, add 160mL of acetone under stirring, cool to 0°C, add 17mL of triethylamine, and heat up to 20°C, stirred and reacted for 11 hours, sampled by HPLC to detect that the residual amount of 7-AVCA was less than 1.0%, then added 120mL of dichloromethane to the reaction solution, stirred for 30 minutes and then stood still for 30 minutes, separated into layers, and the lower dichloromethane phase was reused Extract with 50mL of purified water, stir for 30 minutes, let stand for 30 minutes, separate layers, recover the organic solvent from the lower dichloromethane phase, combine the upper water phase and add 80mL of dichloromethane to wash, stir for 30 minutes, let stand for 30 minutes, and separate layers , the lower dichloromethane phase is used for organic solvent recovery, 2g of activated carbon is added to the upper water phase, stirred and dec...
Embodiment 3
[0038] Add 180mL of purified water, 20.0g7-AVCA and 36g of cefdinir active ester (acetoxyimide) into a 500mL four-necked reaction flask, add 160mL of ethanol under stirring, cool down to 0°C, add 16mL of triethylamine, and heat up to 20°C, stirred and reacted for 10 hours, sampled and detected by HPLC that the residual amount of 7-AVCA was less than 1.0%, then added 120mL dichloromethane to the reaction solution, stirred for 30 minutes and then stood still for 30 minutes, separated into layers, and the lower dichloromethane phase was reused Extract with 50mL of purified water, stir for 30 minutes, let stand for 30 minutes, separate layers, recover the organic solvent from the lower dichloromethane phase, combine the upper water phase and add 80mL of dichloromethane to wash, stir for 30 minutes, let stand for 30 minutes, and separate layers , the lower dichloromethane phase is used for organic solvent recovery, 2g of activated carbon is added to the upper water phase, stirred an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com