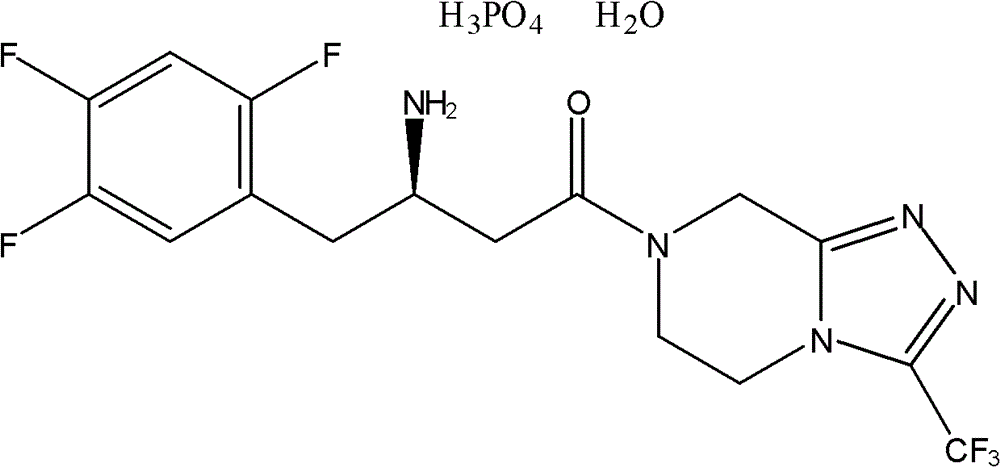
Novel method for synthesizing sitagliptin phosphate and derivative thereof
A technology for sitagliptin phosphate and derivatives, which is applied in the field of synthesizing sitagliptin phosphate and derivatives thereof, and can solve problems such as difficulty in improving ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1) Amino protection
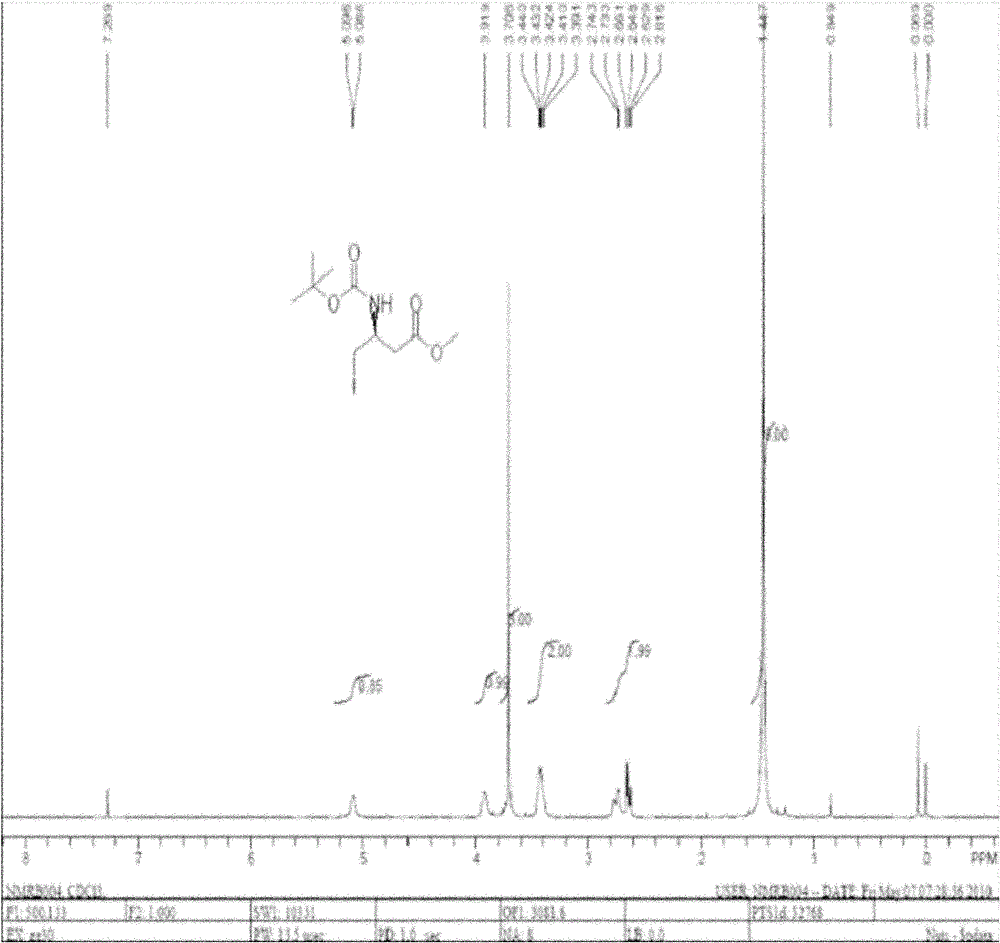
[0052] Add 20g of L-aspartic acid and 200ml of methanol into a three-necked flask equipped with a magnetic stirrer and a thermometer, add 5ml of thionyl chloride dropwise under cooling, stir at room temperature for 5h after the dropwise addition, concentrate under reduced pressure and remove the solvent to obtain solid 4-L - methyl aspartate hydrochloride 40g;
[0053] In a three-necked flask equipped with a magnetic stirrer and a thermometer, add 20 g of 4-L-aspartic acid methyl ester hydrochloride, 20 g of sodium bicarbonate, dissolve in water and 1,4-dioxane, and dissolve Add BOC anhydride, stir overnight, filter with suction, add 2 L of water to the mother liquor, and adjust the pH with hydrochloric acid. Then extracted 3 times with ethyl acetate, dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent to obtain 31 g of light yellow oily liquid N-tert-butoxycarbonyl-4-L-aspartic acid methyl ester;
[005...
Embodiment 2
[0066] 1) Amino protection
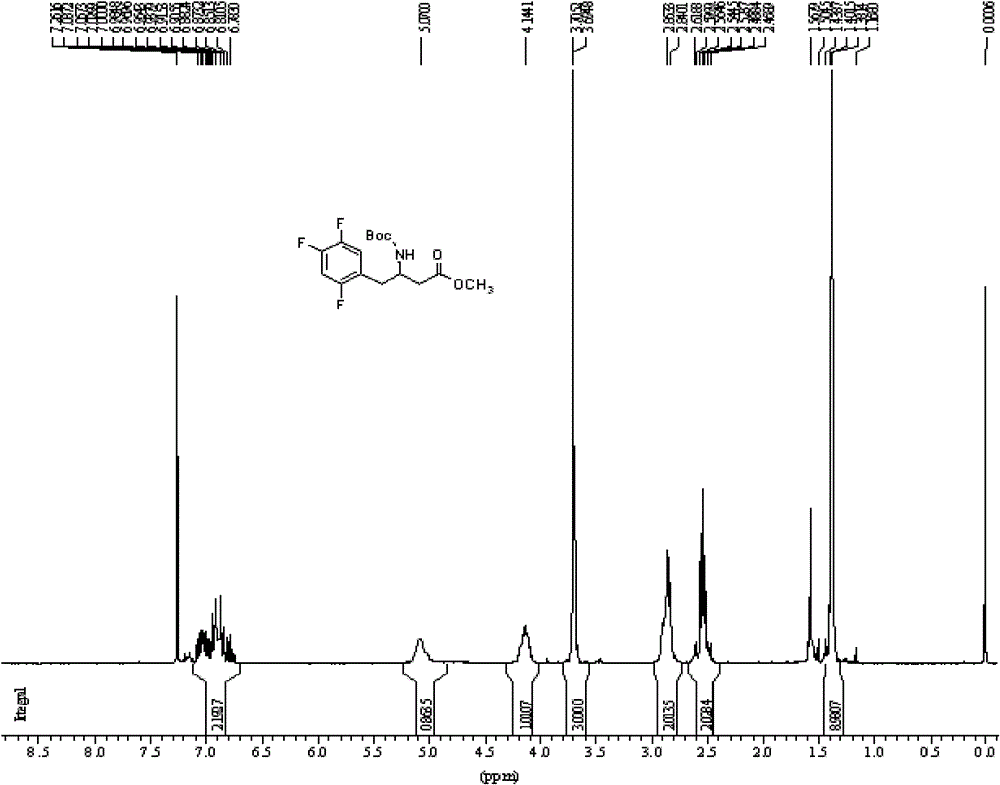
[0067] Add 22g of L-aspartic acid and 250ml of ethanol to a three-necked flask equipped with a magnetic stirrer and a thermometer, add 12ml of thionyl chloride dropwise under cooling, stir at room temperature for 5 hours after the dropwise addition, concentrate under reduced pressure and remove the solvent to obtain the solid 4-L - ethyl aspartate hydrochloride 40g;
[0068] In a three-necked flask equipped with a magnetic stirrer and a thermometer, add 22 g of 4-L-ethyl aspartic acid hydrochloride, 28 g of sodium bicarbonate, dissolve in water and 1,4-dioxane, and dissolve Add BOC anhydride, stir overnight, filter with suction, add 2 L of water to the mother liquor, and adjust the pH with hydrochloric acid. Then extracted 3 times with ethyl acetate, dried over anhydrous sodium sulfate, concentrated under reduced pressure and desolventized to obtain 37 g of light yellow oily liquid N-tert-butoxycarbonyl-4-L-aspartic acid ethyl ester;
[0069] 2) ...
Embodiment 3
[0081] 1) Amino protection
[0082] Add 20g of L-aspartic acid and 200ml of methanol into a three-necked flask equipped with a magnetic stirrer and a thermometer, add 5ml of thionyl chloride dropwise under cooling, stir at room temperature for 5h after the dropwise addition, concentrate under reduced pressure and remove the solvent to obtain solid 4-L - methyl aspartate hydrochloride 40g;
[0083] In a three-necked flask equipped with a magnetic stirrer and a thermometer, add 20 g of 4-L-aspartic acid methyl ester hydrochloride, 20 g of sodium bicarbonate, dissolve in water and 1,4-dioxane, and dissolve Add BOC anhydride, stir overnight, filter with suction, add 2 L of water to the mother liquor, and adjust the pH with hydrochloric acid. Then extracted 3 times with ethyl acetate, dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent to obtain 31 g of light yellow oily liquid N-tert-butoxycarbonyl-4-L-aspartic acid methyl ester;
[008...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com