Fibrous mesoporous alumina and preparation method thereof
A technology of mesoporous alumina, which is applied in the field of batch synthesis of fibrous mesoporous alumina, can solve the problems of unsuitability for industrialization, complex operating conditions, and low production cost, and achieve low production cost, simple process, and large specific surface area. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
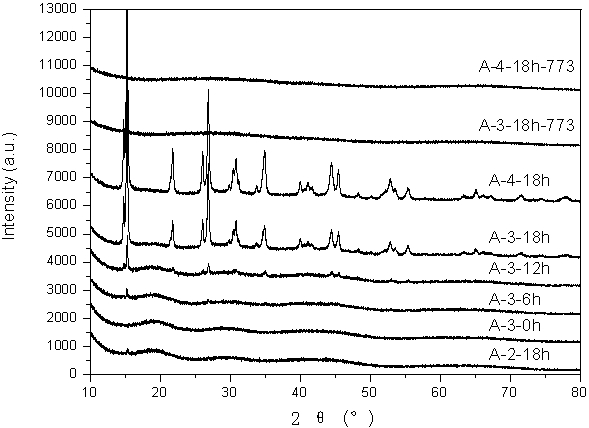
[0029] At 343 K, 2.32 g of polyoxyethylene-polyoxypropylene-polyoxyethylene block copolymer (P123) was dissolved in 50 mL of aluminum nitrate aqueous solution (0.5 mol / L). Under stirring, 150 mL of ammonium carbonate aqueous solution (0.5 mol / L) was added dropwise, and stirred evenly. The obtained inorganic aluminum precipitates were aged at 343 K for 0 h, 6 h, 12 h and 18 h, respectively. The resulting product was washed and filtered with hot water until neutral, and dried at 323 K to obtain an inorganic aluminum precursor. The precursor was calcined at 773 K for 4 h to obtain alumina powder. The samples are labeled A-3-0h, A-3-6h, A-3-12h and A-3-18h, respectively. The resulting inorganic aluminum precursor is characterized by XRD (see figure 1 ). The results showed that with the increase of aging time, the crystal phase of the inorganic aluminum precursor changed from Gibbsite to ammonium aluminum carbonate (NH 4 Al(OH) 2 CO 3 ). Combined with SEM characterization,...
Embodiment 5~8
[0031] The implementation method is the same as in Example 4, except that the addition amount of ammonium carbonate aqueous solution (0.5 mol / L) is 100 mL, 200 mL, 300 mL and 400 mL respectively. When the ammonium carbonate aqueous solution was added in an amount of 100 mL, the crystal phase of ammonium aluminum carbonate could not be obtained, nor could the fibrous morphology be formed. When the addition amount of ammonium carbonate aqueous solution is 200 mL, 300 mL and 400 mL, the crystal phase of ammonium aluminum carbonate can be obtained, and the fibrous morphology can be formed at the same time.
Embodiment 9~13
[0033] At 343 K, no surfactant is added or the ratio of aluminum nitrate to surfactant (molar ratio) is 1:0.02, the surfactant cetyltrimethylammonium bromide, sodium lauryl sulfate And polyethylene glycol 2000 and a mixture of cetyltrimethylammonium bromide and sodium lauryl sulfate (molar ratio 1:1) were dissolved in 50 mL of aluminum nitrate aqueous solution (0.5 mol / L). Under stirring, 150 mL of ammonium carbonate aqueous solution (0.5 mol / L) was added dropwise, and stirred evenly. The obtained inorganic aluminum precipitate was aged at 343 K for 18 h. The resulting product was washed and filtered with hot water until neutral, and dried at 323 K to obtain an inorganic aluminum precursor. The precursor was calcined at 773K for 4 h to obtain alumina powder. Samples were labeled A-NON, A-CTAB, A-SDS, A-PEG2000 and A-CTAB+SDS. Calcined at 773 K, the fibrous structure is preserved.
[0034] Table 1. Examples of structural and performance characterization of fibrous mesoporou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com