Use of an oxyhydroxy salt related to the family of lamellar double hydroxides for the design and manufacture of an electrode with a view to storing electrical energy
A technology of hydroxide and iron oxyhydroxide salt, which is applied in the manufacture of hybrid/electric double layer capacitors, iron oxide/iron hydroxide, nickel compounds, etc., and can solve problems such as huge energy performance and polluting waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
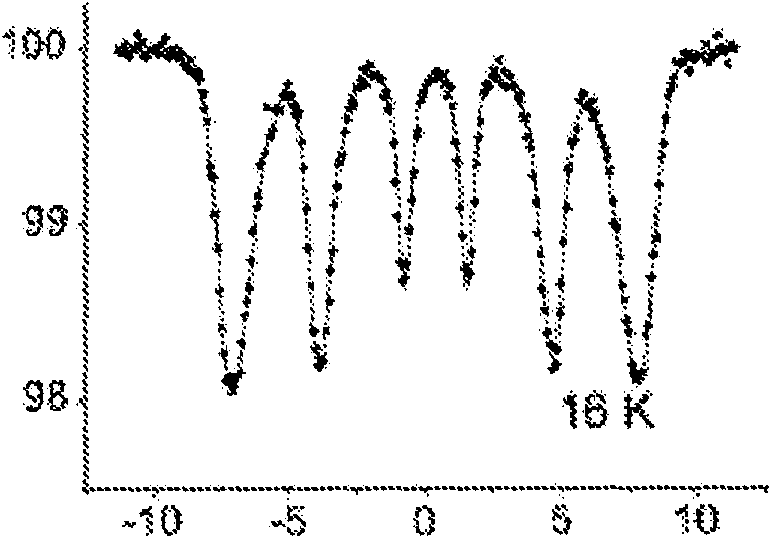
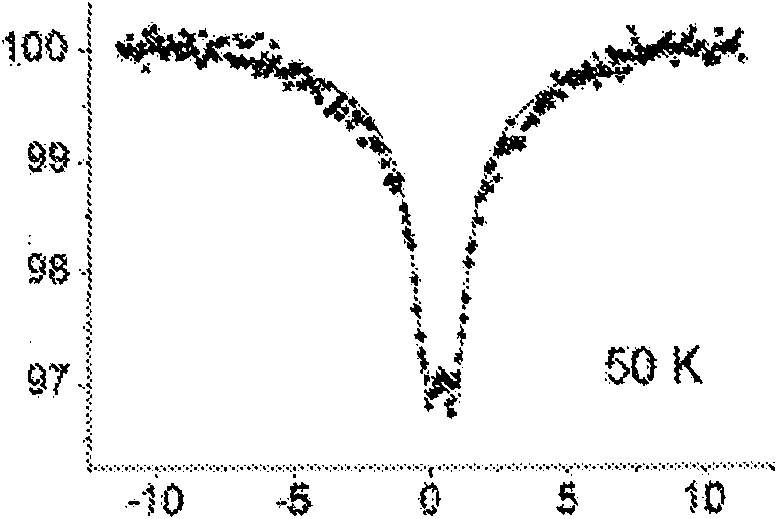
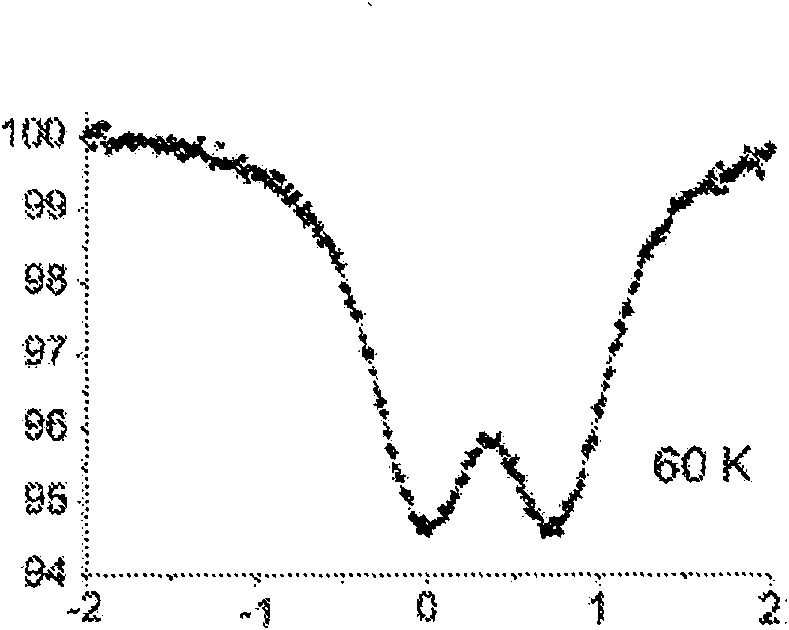
[0201] Example 1: Mössbauer spectroscopy
[0202]Mössbauer reflectance spectroscopy using a MIMOS device was used to determine the oxidation state of iron in composites and films. MIMOS devices work by detecting the backscattered geometry of radiations réémises (14.4 keV for gamma rays and 6.4 keV for X-rays). MIMOS was also used to study iron compounds present on the soil of the planet Mars. (NASA and European Space Agency)
[0203] Mössbauer spectra were calibrated at ambient temperature with iron plates and adjusted with Lorentzian lines and single ferric quadrupole doublets.
Embodiment 2
[0204] Embodiment 2: the preparation of material
[0205] Preparation of stoichiometric ferrous-ferric hydroxycarbonates by chemical synthesis [Fe II 4 Fe III 2 (OH) 12 ] 2+ CO 3 2- GR (CO 3 2- ), where Fe(OH) 2 Oxidative preparation of precipitates in the presence of carbonate ions, or by Fe as described by Ruby et al. (2006, Geoscience) II and Fe III Co-precipitation preparation of ions in the presence of anions.
[0206] The ferrous-ferric hydroxycarbonate is then treated with an excess of a strong oxidizing agent such as H 2 o 2 (GR * (1)) or dry in air (GR * (2)) is completely deprotonated, as described by Génin et al. (2006, Geoscience), resulting in the formula [Fe III 6 o 12 h 8 ] 2+ CO 3 2- Ferric oxycarbonate (GR * (1) or (2)).
[0207] The obtained products were characterized by X-ray diffraction, Mössbauer spectroscopy, vibrational spectroscopy (Raman or infrared) and transmission electron microscopy.
[0208] GR (CO 3 2- ) and GR * The ...
Embodiment 3
[0218] Embodiment 3: the preparation of composite material
[0219] Hydroxyoxycarbonate (GR * (1)) and carbon powder are mixed with paraffin or oil as a binder. In the case of paraffin, by mixing 250 mg carbon and 50 or 100 mg GR * (1) A composite material was prepared, and then the mixture was heated in the presence of 100 mg of paraffin to bond the whole.
[0220] With 50 or 100mg GR * (1) The Mössbauer spectrum of the obtained composite material ( Figure 6A with 6B ) and GR * (1) quite.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com