Bt protein in-vitro insect resistance identification method
A technology of bt protein and carrier, which is applied in the field of biological genetic engineering to achieve the effect of not easy to mold, efficient expression, maintaining integrity and biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 A method for identifying Bt protein against insects in vitro, comprising the following steps:
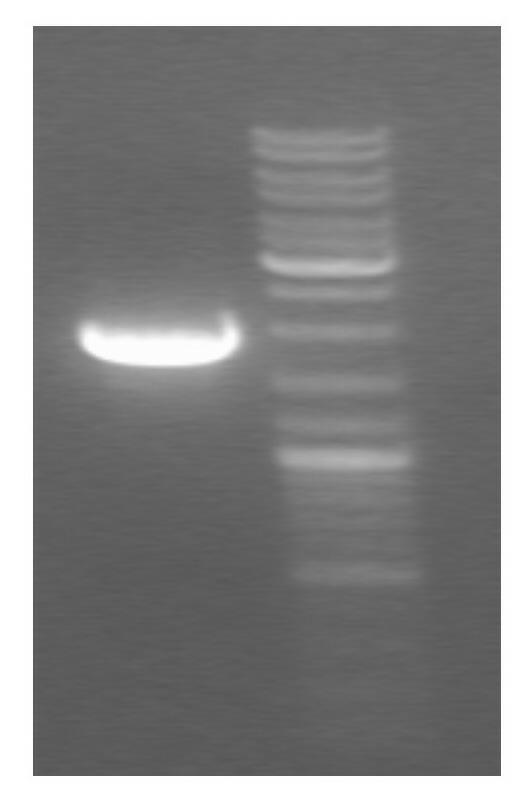
[0042] (1) Transform and synthesize the insect-resistant gene Bt ( figure 1) into the prokaryotic expression vector pET-28B (purchased from Shanghai Biological Engineering Co., Ltd.); the specific method is as follows:
[0043] Step 1: Digest pUC with restriction enzymes bt , the gel recovery kit recovers and purifies the Bt fragment;
[0044] Step 2: Digest pET28b with the same restriction enzyme, and recover and purify the digested pET28b fragment with the gel extraction kit;
[0045] Step 3: Ligate the two purified fragments, and construct the resulting prokaryotic expression plasmid named pET28b bt , identified by restriction endonuclease digestion, indicating that the vector was constructed correctly ( figure 2 );
[0046] (2) Transform the prokaryotic expression vector PET-28B containing the Bt gene into Escherichia coli BL21 (purchased from Shangh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com