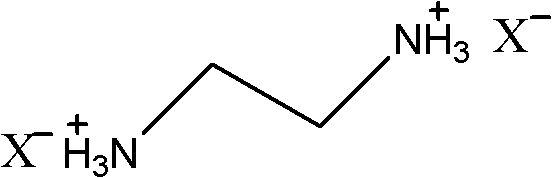
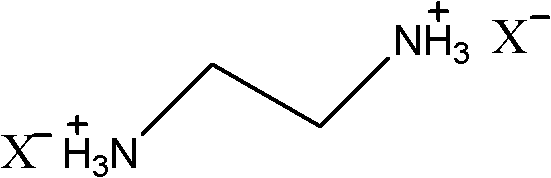
Ethylenediamine-nitrophenol type ionic liquids and preparation method thereof
A technology of ionic liquid and dinitrophenol, which is applied in the preparation of amino compounds, chemical instruments and methods, and the preparation of organic compounds, can solve problems such as production and people's lives, national security threats, safety accidents, and loss of raw materials, and achieve compatibility Good performance, high structural symmetry, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Firstly, 0.04 mol of 2-nitrophenol was weighed and dissolved in 50 ml of ethanol, and 0.01 mol of ethylenediamine was weighed and dissolved in 10 ml of ethanol. Slowly add the ethylenediamine ethanol solution dropwise to the stirred nitrophenol ethanol solution at 30°C, and the dropwise addition is completed in 30 minutes, and a yellow precipitate is formed during the dropwise addition. After the dropwise addition, the reaction was continued at 30°C for 30 minutes, filtered and dried at 40°C to obtain 3.11 g of a yellow product, with a yield of 92%. The proton magnetic spectrum data of gained compound is: δ=2.81 (s, CH2, 4H), 6.44 (t, CH, 2H), 6.78 (d, CH, 2H), 7.24 (t, CH, 2H), 7.74 ( d, CH, 2H) ppm.
Embodiment 2
[0015] Firstly, 0.04 mol of 3-nitrophenol was weighed and dissolved in 50 ml of isopropanol, and 0.01 mol of ethylenediamine was weighed and dissolved in 10 ml of isopropanol. Slowly add the ethylenediamine isopropanol solution dropwise to the stirred nitrophenol isopropanol solution at 5-10°C, and the dropwise addition is completed within 30 minutes. Then, the reaction was continued at 10°C for 60 min. During the reaction, a precipitate gradually precipitated out, and was filtered and dried at 50°C to obtain 1.02 g of a yellow product with a yield of 30%. The proton magnetic spectrum of gained compound is: δ=2.65 (s, CH2, 4H), 5.38 (s, NH3, 6H), 7.12 (d, CH, 2H), 7.37 (t, CH, 2H), 7.46 (s , CH, 2H), 7.50 (d, CH, 2H) ppm.
Embodiment 3
[0017] Firstly, 0.04 mol of 2,4-nitrophenol was weighed and dissolved in 80 ml of ethanol, and 0.017 mol of ethylenediamine was weighed and dissolved in 10 ml of ethanol. Slowly add the ethylenediamine ethanol solution to the stirring nitrophenol ethanol solution at 50°C, and the dropwise addition is completed in 30 minutes, during which a yellow precipitate is formed. After the dropwise addition, the reaction was continued at 50° C. for 30 minutes, filtered and dried at 30° C. to obtain 6.98 g of a yellow product with a yield of 96%. The proton magnetic spectrum of the obtained compound is: δ=1.25 (s, CH2, 4H), 4.65 (d, CH, 2H), 6.01 (d, CH, 2H), 6.78 (s, CH, 2H) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com