Catalyst, method for synthesizing (S)-octopamine and method for synthesizing (S)-N-trans-feruloyloctopamine
A synthesis method and catalyst technology, which are applied in the synthesis field of -N-trans-feruloyl-desmethylsypheline, can solve the problems of high requirements for synthesis conditions, complicated operations, numerous steps, etc., and improve synthesis efficiency and yield. high efficiency, high catalytic efficiency, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0085] The present invention also provides a kind of synthetic method of (S)-octopamine, comprising:
[0086] a), under the action of the catalyst described in the above technical scheme, p-Hydroxybenzaldehyde and nitromethane react to generate (S)-octopamine precursor;
[0087] b) Under the action of a palladium-carbon catalyst, the (S)-octopamine precursor undergoes a hydrogenation reduction reaction with hydrogen to generate (S)-octopamine.
[0088] The catalyst described in the above technical scheme catalyzes the reaction between p-hydroxybenzaldehyde and nitromethane to generate (S)-octopamine precursor, and the reaction formula is as follows:
[0089]
[0090] The precursor of (S)-octopamine is (S)-1-(4-hydroxyphenyl)-2-nitroethanol.
[0091] In the reaction of generating (S)-octopamine precursor, the molar ratio of the ligands in the p-hydroxybenzaldehyde, nitromethane and catalyst is preferably 1:2~20:0.01~0.3, more preferably 1: 5-15:0.05-0.3, most preferably 1:...
Embodiment 1
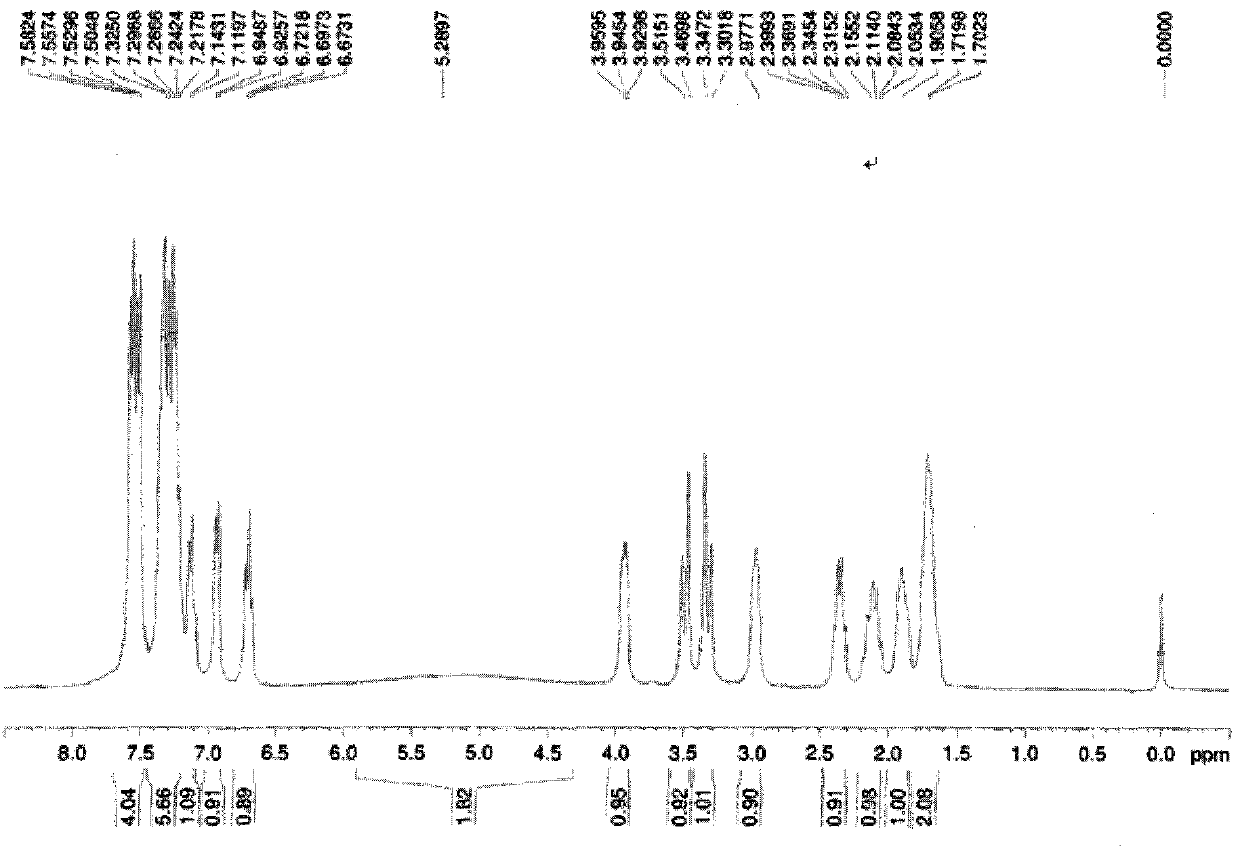
[0107] Under argon protection, 2.533g (10mmol) of commercially available (S)-diphenylprolinol, 1.901g (10mmol) of commercially available 2-hydroxy-3-trifluoromethyl- Benzaldehyde, 20mL ethanol and a stirring magnet, stir at room temperature, track the reaction until the amine disappears, add 378.3mg (10mmol) NaBH 4 , After stirring at room temperature for 24h, the reaction was quenched by adding 1mol / L HCl, and then saturated NaHCO 3 The reaction mixture was neutralized to a pH value of 7, extracted 3 times with 20 mL of ethyl acetate, and the resulting organic phase was dried over anhydrous sodium sulfate, spin-dried and passed through a silica gel column, and the mobile phase was petroleum with a volume ratio of 1:6. ether and ethyl acetate to give 3.42 g of product in 80% yield, [α] D 25 +91.0 (c 1.18, CH 2 Cl 2 ).
[0108] Carry out nuclear magnetic resonance to described product, the result sees figure 1 and figure 2 , figure 1 The H NMR spectrum of the ligand pr...
Embodiment 2
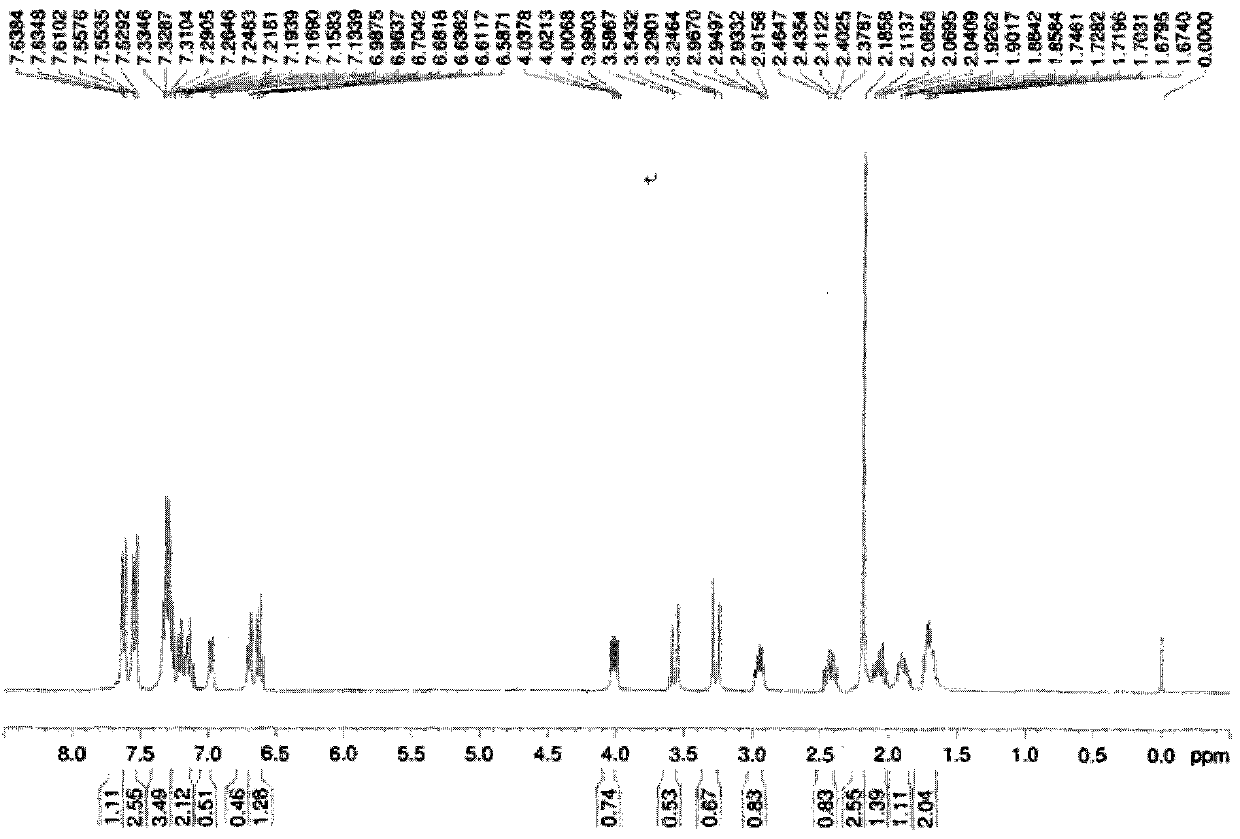
[0110] Under argon protection, add 2.533g (10mmol) commercially available (S)-diphenylprolinol, 1.362g (10mmol) commercially available 2-hydroxyl-3-methyl-benzaldehyde to a 50mL round bottom bottle , 20mL ethanol and a stirring magnet, stirred at room temperature, tracked the reaction until the amine disappeared, added 378.3mg (10mmol) NaBH 4 , After stirring at room temperature for 24h, the reaction was quenched by adding 1mol / L HCl, and then saturated NaHCO 3 The reaction mixture was neutralized to a pH value of 7, extracted 3 times with 20 mL of ethyl acetate, and the resulting organic phase was dried over anhydrous sodium sulfate, spin-dried and passed through a silica gel column, and the mobile phase was petroleum with a volume ratio of 1:8. ether and ethyl acetate to give 2.801 g of product, yield 75%, [α] D 25 +61.2 (c 0.92, CH 2 Cl 2 ).
[0111] Carry out nuclear magnetic resonance to described product, the result sees image 3 and Figure 4 , image 3 The H NM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com