In vitro detection method of hepatitis B virus C antibody
A hepatitis B virus, in vitro detection technology, applied in measurement devices, color/spectral property measurement, instruments, etc., can solve problems such as the inability to meet the needs of large-scale in vitro detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1Protein A is coated on microtiter plate
[0067]Select an untreated polystyrene microtiter plate, add 0.9μg / ml Protein A dissolved in carbonate buffer, pH9.2-9.8, 100μl / well, overnight at 4°C. The coating solution was removed, and 200 μl of 1% (w / v) BSA solution (PBS, pH 7.4) was added to each well for blocking, at 37° C. for 1-2 hours.
Embodiment 2
[0068] Embodiment 2 Serum sample detection method
[0069] Wash the microtiter plate 1-2 times with PBST (PBS buffer containing 0.05% Tween-20), and pat dry each time.
[0070] After adding 90 μl of PBS to each well, add 10 μl of serum to be tested into each well, mix well and incubate at 37° C. for 60 minutes.
[0071] After the reaction solution was removed and patted dry, the plate was washed 4 times with PBST, each time patted dry.
[0072] Add 40 μl of recombinant HBcAg (SEQ ID NO: 1, provided by Shanghai Rongsheng Biopharmaceutical Co., Ltd.) (diluted 1:2000 with PBS) and 40 μl of HRP-labeled HBcAg antibody (diluted 1:2000 with PBS) into each well.
[0073] Add 50 μl each of substrate A and B solution to each well, mix well, incubate at 37°C for 15 minutes, then add stop solution, and measure with a 450nm microplate reader.
[0074] Substrate A solution preparation:
[0075] Weigh 17.9g of citric acid and Na 2 HPO 4 12 2 O 4.67g was dissolved in 400ml deionized wat...
Embodiment 3
[0081] Embodiment 3HBcAb detects parallel control
[0082] The currently used HBcAb competition ELISA detection kit (Shanghai Rongsheng Biopharmaceutical Co., Ltd.) was used for comparison.
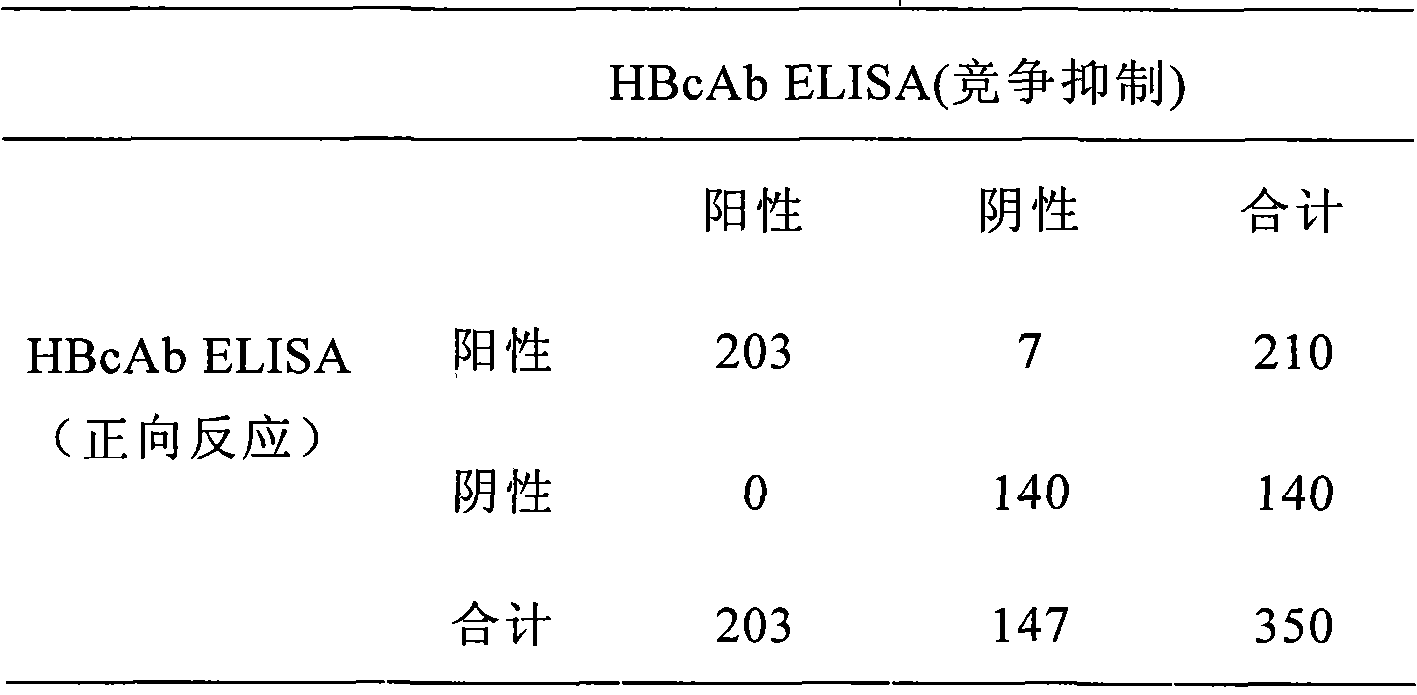
[0083] A total of 350 specimens were used in this experiment, among which,
[0084] Da San Yang (positive for HBsAg, HBeAg and HBcAb): 110 cases;
[0085] Small Sanyang (positive for HBsAg, HBeAb and HBcAb): 100 cases;
[0086] Healthy human serum (from blood bank): 140 cases.
[0087] The test results are shown in Table 1.
[0088] Table 1 HBcAb detection parallel control
[0089]
[0090] It can be obtained from Table 1:
[0091] Positive consistency rate: 203 / 203=100%;
[0092] Negative consistency rate: 140 / 147=95.2%;
[0093] Overall agreement rate: 343 / 350=98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com