Method for producing cephalothin acid by water phase method
A technology of cefotaxime acid and production method, applied in directions such as organic chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
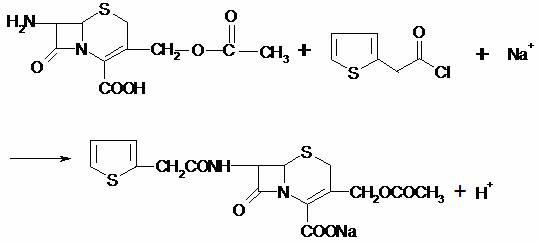
[0053] A production process for producing cefotaxime acid by an aqueous phase method. Its process flow chart is shown in Figure 1, and it is carried out according to the following steps in turn:
[0054] 1) React
[0055]At room temperature, add in sequence: deionized water 300L, 7-ACA 50Kg, organic solvent ethyl acetate 45L; then add about 40.0Kg of alkaline regulator sodium carbonate at room temperature within a certain period of time, and stir to 7- ACA dissolves completely. Cool the temperature of the system to about 10°C, and add about 20 L of 2-thiophene acetylation reagent 2-thiophene acetyl bromide within 150 min. After adding the material, time the reaction for 30 minutes. After the reaction is completed, add the reaction terminator sodium metabisulfite 5.0Kg , heat up to a certain temperature, add about 60.0Kg of sodium chloride, a precipitation accelerator, and let stand for 30 minutes to precipitate cefotaxime sodium; perform suction filtration at room temper...
Embodiment 2
[0064] A production technique for producing cefotaxime acid by an aqueous phase method is carried out successively according to the following steps:
[0065] At room temperature, add in sequence: 650L of deionized water, 100Kg of 7-ACA, and 100L of petroleum ether; then add about 85.0Kg of sodium bicarbonate at room temperature within a certain period of time, and stir until the 7-ACA is completely dissolved. Cool the temperature of the system to about 15 ℃, add about 45 L of 2-thiophene acetyl chloride within 120 min, after adding the material, time the reaction for 30 minutes, add 10.0Kg of sodium bisulfite after the reaction is completed, and add bromine at the temperature of 20 ℃. About 125.0Kg of sodium chloride, let stand for 30min to precipitate cefotaxime sodium; suction filtration at 10°C, and wash twice with petroleum ether totaling 165L to obtain cefotaxime sodium wet product.
Embodiment 3
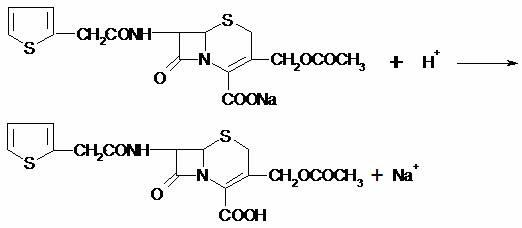
[0067] The cefotaxime sodium wet product obtained in Example 2 was dissolved in 600 L of purified water at room temperature, 5.0 Kg of medical activated carbon was added, stirred for 30 min, suction filtered, and the filter and filter residue were washed with 120 L of purified water and 75 L of ethyl acetate, and collected. filtrate.
[0068] Collect the purified and decolorized filtrate, adjust the pH of the system to about 2.4-2.6 with hydrochloric acid at room temperature, stir and grow the crystals for 1 hour, cool and suction filtration, and wash the wet product with a total of 250L of purified water, take out the wet product and put it in the Vacuum dry at +42±2°C for 20-22 hours until H 2 O≤0.5%, about 64.0-68.0Kg of cefotaxime acid product is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com