Valsartan amlodipine capsule and preparation method thereof
A technology of valsartan and amlodipine capsules and amlodipine besylate, which is applied in the technical field of improvement of oral solid preparations, and can solve the problems of slow release, inability to solve disintegration and dissolution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
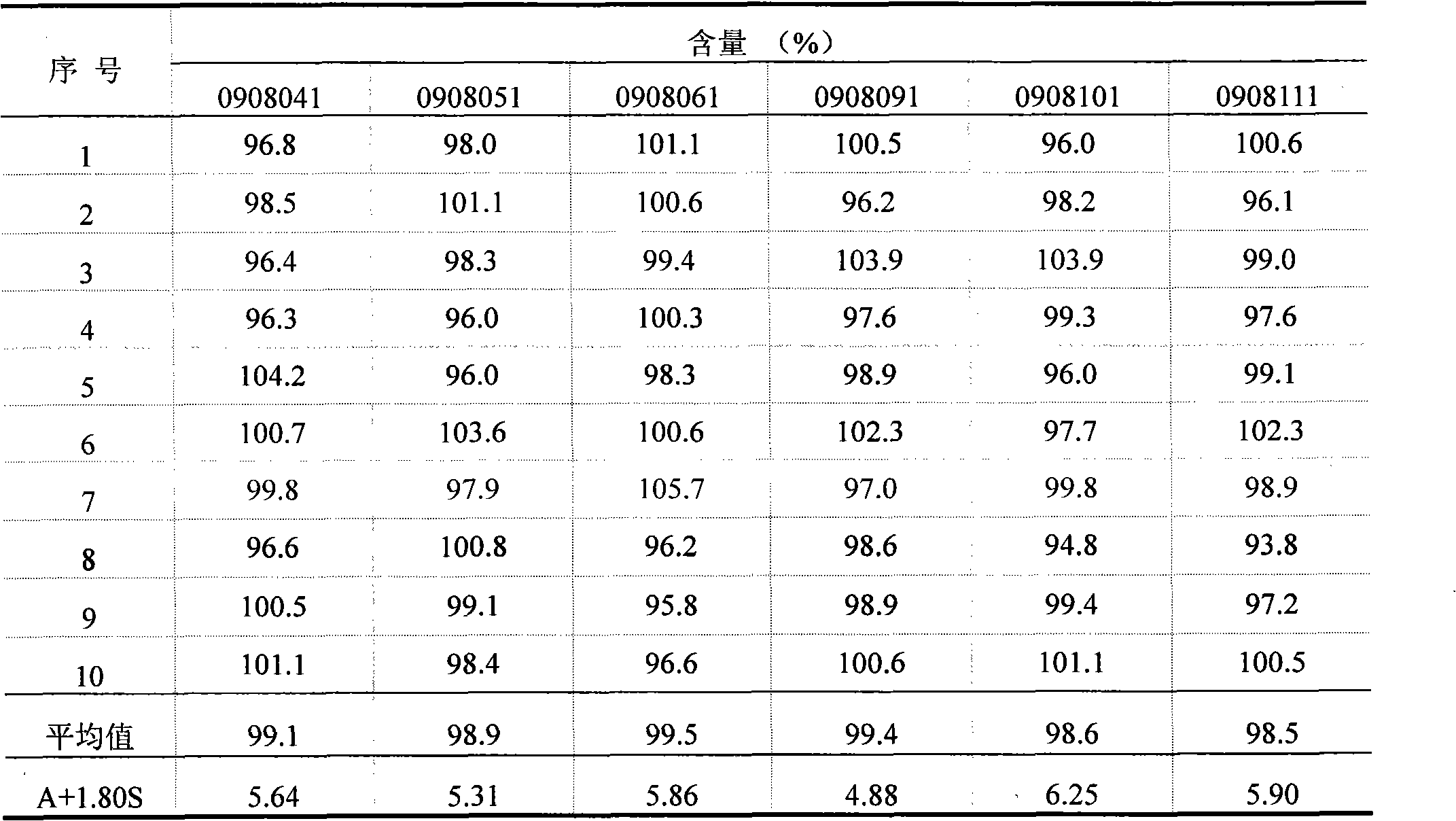
Examples
Embodiment 1
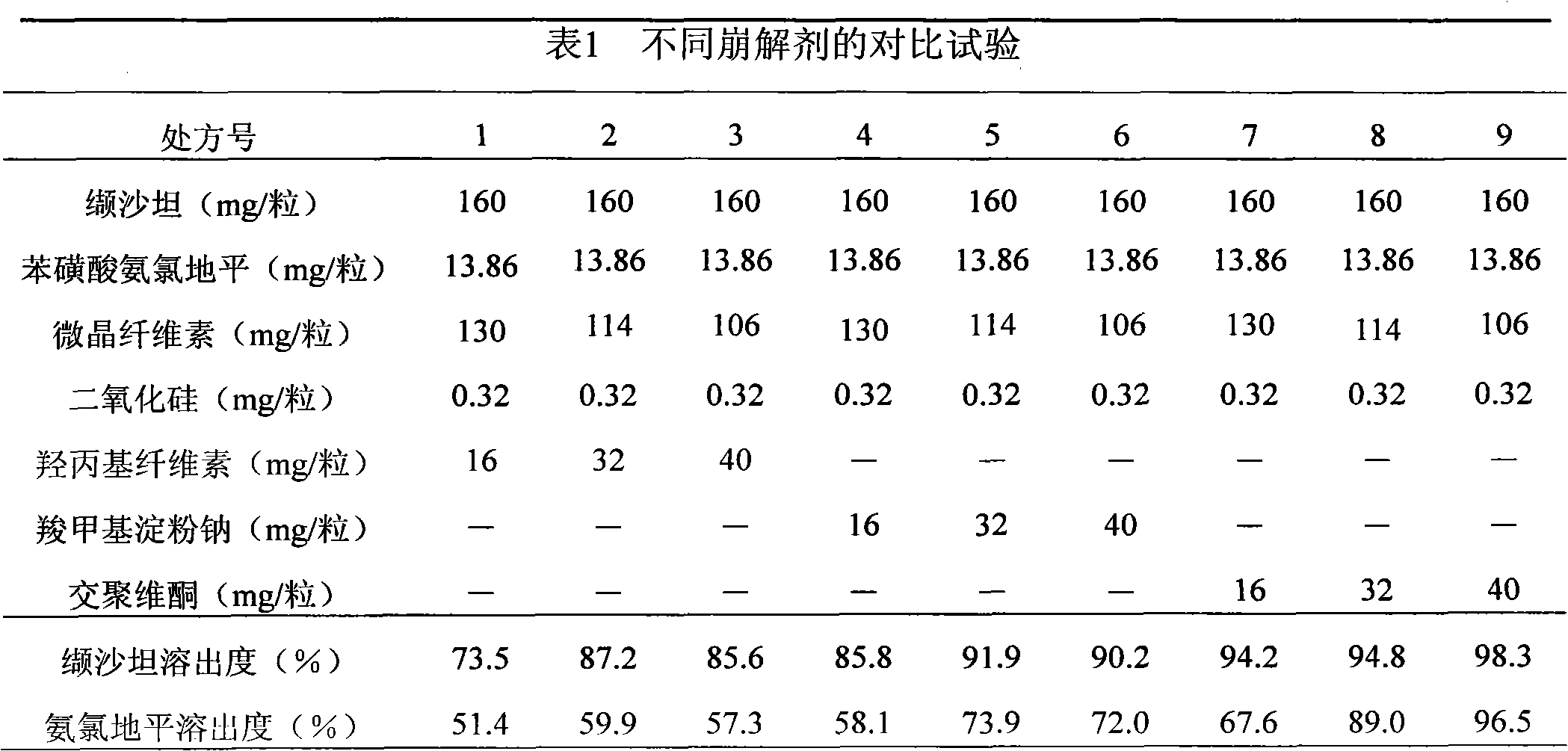
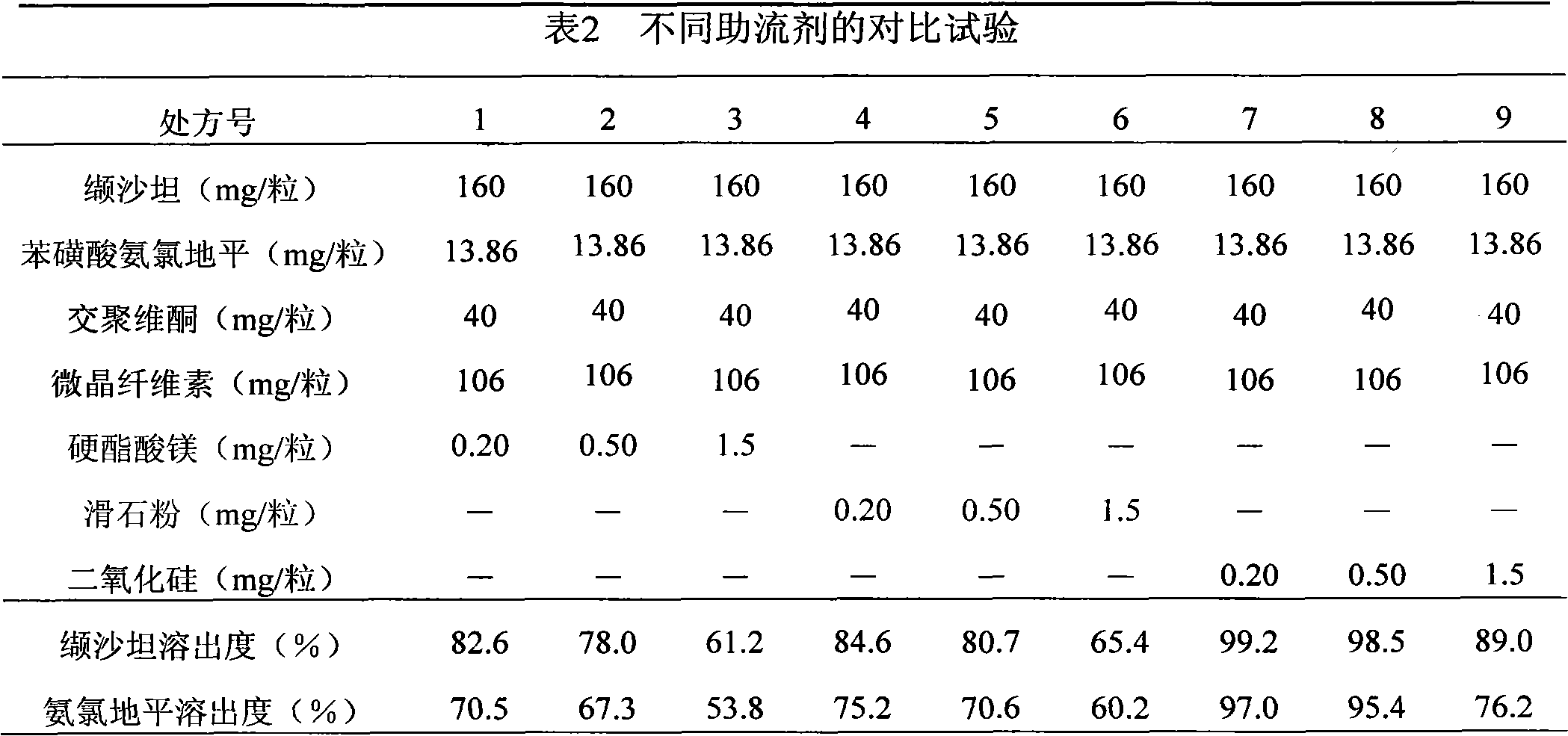
[0032] Valsartan 160g
[0033] Amlodipine besylate 13.86g
[0034] Microcrystalline Cellulose 106g
[0035] Crospovidone 50g
[0036] Silica 0.65g
[0037]
[0038] Makes 1000 capsules
[0039] Amlodipine besylate, crospovidone, microcrystalline cellulose, valsartan, and silicon dioxide in the prescribed amount are added to the mixer in sequence, fully mixed, and filled into capsules.
Embodiment 2
[0041] Valsartan 160g
[0042] Amlodipine besylate 6.93g
[0043]Microcrystalline Cellulose 50g
[0044] Crospovidone 40g
[0045] Silica 0.26g
[0046]
[0047] Makes 1000 capsules
[0048] Amlodipine besylate, crospovidone, microcrystalline cellulose, valsartan, and silicon dioxide in the prescribed amount are added to the mixer in sequence, fully mixed, and filled into capsules.
Embodiment 3
[0050] Valsartan 320g
[0051] Amlodipine besylate (calculated as amlodipine) 13.86g
[0052] Microcrystalline Cellulose 60g
[0053] Crospovidone 80g
[0054] Silica 1.0g
[0055]
[0056] Makes 1000 capsules
[0057] Amlodipine besylate, crospovidone, microcrystalline cellulose, valsartan, and silicon dioxide in the prescribed amount are added to the mixer in sequence, fully mixed, and filled into capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com