Avian influenza virus H9 subtype positive blood serum and negative blood serum standard substances and preparation methods thereof
A bird flu virus and standard material technology, applied in antiviral immunoglobulin, immunoglobulin from serum, biological testing, etc., can solve problems such as no technical specifications to follow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Production of positive serum and inspection of semi-finished products
[0122] 1. Positive serum production
[0123] (1) For immunization, select SPF chickens aged 3 to 4 months and raise them in a negative pressure isolator, and use the prepared avian influenza inactivated vaccine to vaccinate twice by chest intramuscular injection. After 21 days, the second inoculation was carried out, the dose was 1.5ml / only, and the injection was divided into points.
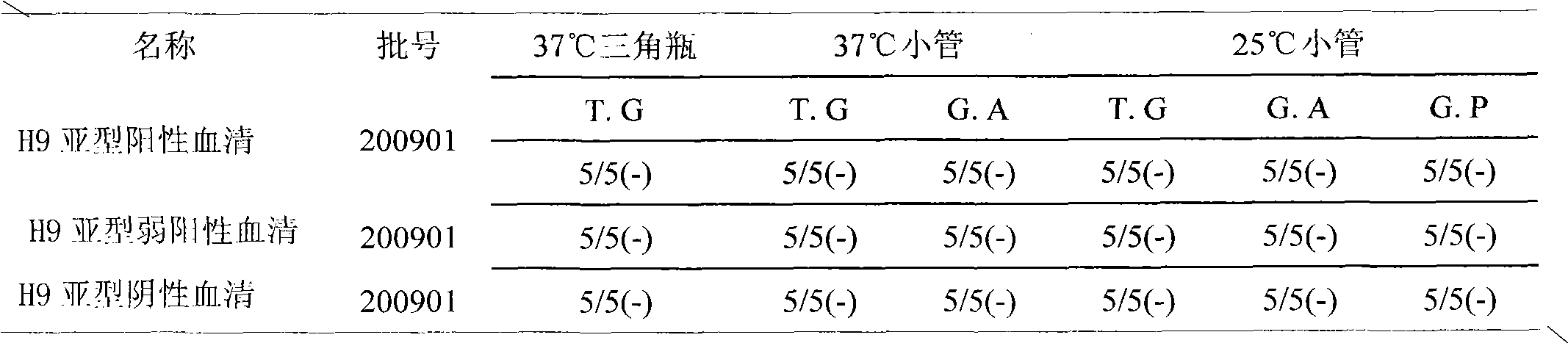
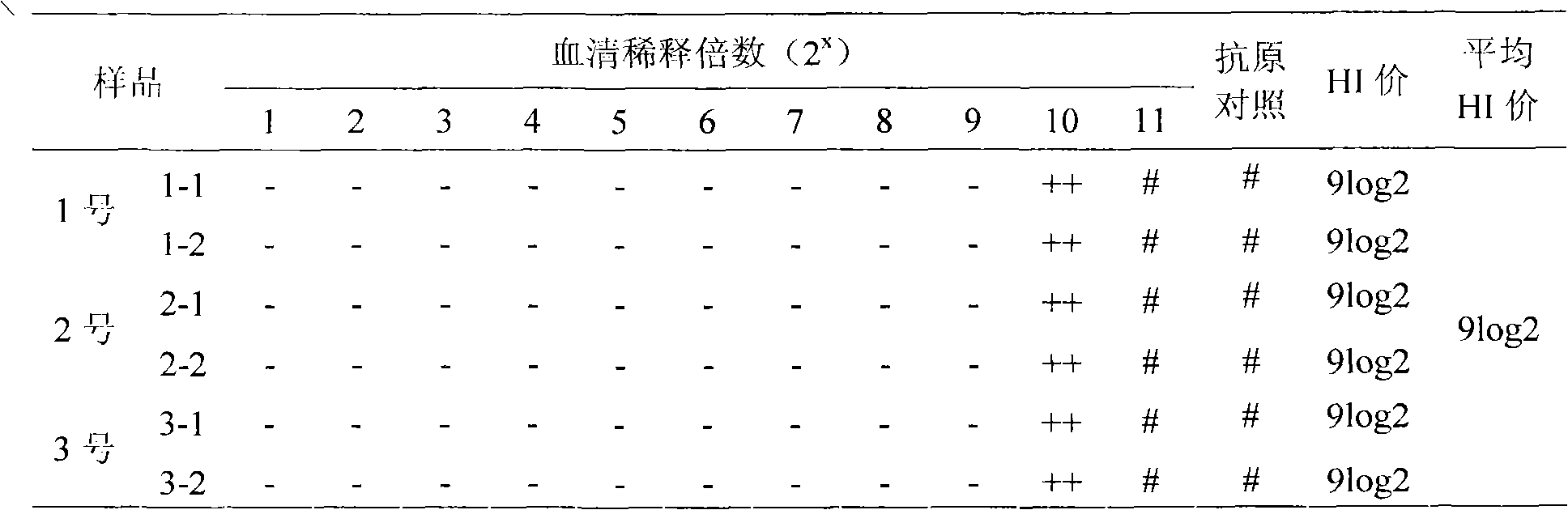
[0124] (2) Blood test: 28 days after the second immunization, the serum was collected by means of subwing vein blood collection. At the same time, the chicken and serum were numbered correspondingly, and the HI antibody titer was determined respectively (according to the "Appendix"), and the HI antibody was selected. SPF chickens with a titer ≥ 8log2 are used for standby.
[0125] (3) Separation of serum Select SPF chickens with HI antibody titer ≥ 8log2 Use a 20ml sterile syringe to collect blood from the heart in ...
Embodiment 2
[0133] Production of weak positive serum and inspection of semi-finished products
[0134] 1. Weak positive serum production
[0135] (1) Immunization SPF chickens aged 3 to 4 months were selected and raised in a negative pressure isolator, and the prepared inactivated avian influenza vaccine was vaccinated by chest intramuscular injection, and the inoculation dose was 0.5ml / bird.
[0136] (2) Blood test: 14 to 21 days after immunization, the serum was collected by subwing vein blood collection, and the chicken and serum were correspondingly numbered at the same time, and the HI antibody titer was determined respectively (according to the "Appendix"), and the HI antibody titer was selected. SPF chicken with a price of 5log2 is reserved.
[0137] (3) Separation of serum Select SPF chickens with HI antibody titer 5log2 and use a 20ml sterile syringe to collect blood from the heart in a negative pressure isolator. After the blood is collected, place it at 37°C for 4 hours, and t...
Embodiment 3
[0145] Manufacture of negative serum and inspection of semi-finished products
[0146] 1. Negative Serum Production
[0147] The young SPF chickens were raised in the negative pressure isolator in the biosafety animal laboratory, and the heart blood was collected with a sterile syringe in the negative pressure isolator in the biosafety animal laboratory. After the blood was collected, it was placed at 37°C for 4h. Then put it in the refrigerator at 4°C overnight, extract the serum in the ultra-clean workbench in the sterile room, and then mix it in the same sterilized container.
[0148] 2. Inspection of negative serum semi-finished products
[0149](1) Sterility test Take samples of the mixed serum and test according to "Chinese Veterinary Pharmacopoeia", and there should be no growth of bacteria or mold.
[0150] (2) Titer determination The HI antibody titer should be determined according to the appendix, and it should be negative.
[0151] (3) Specificity Under the same ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com