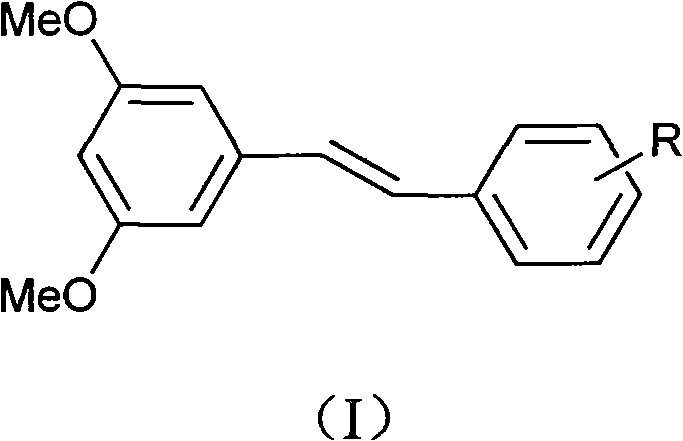
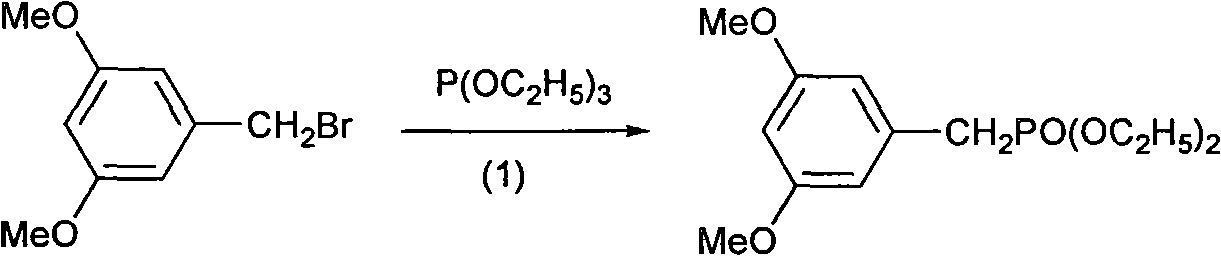3,5-dimethoxystilbene derivative, preparation method and application thereof in anti-drug resistant bacteria
A technology of dimethoxystilbene and stilbene, which is applied in the direction of antibacterial drugs, the preparation of organic compounds, and the preparation of sulfonates, can solve the problems of multi-drug resistance of various antibiotics and the difficulty of selecting antibiotics, and achieve The effect of good industrialization prospect, reduction of reaction cost and optimization of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of (E)-3,5-dimethoxy-2'-nitro-1,2-stilbene:
[0030] Dissolve 10g (0.043mol) of 3,5-dimethoxybenzyl bromide in 7.9g (0.047mol) of triethyl phosphite, heat to 115°C, stir and reflux for 5 hours, distill off excess triethyl phosphite under reduced pressure The ester yielded 11.2 g of 3,5-dimethoxybenzyl phosphonate as an orange-red oil, which was directly used in the next reaction.
[0031] Add 11.2g (0.04mol) of orange-red oily 3,5-dimethoxybenzylphosphonate and 6g (0.04mol) of 2-nitrobenzaldehyde to 55ml of N,N-dimethyl After stirring and dissolving in formamide, add dropwise 24ml of 15% by mass potassium tert-butoxide (0.032mol) in absolute ethanol solution, stir for 8h at room temperature, pour the reaction solution into ice water, filter the precipitated solid, and dry , Recrystallized with a mixture of ethyl acetate and n-hexane with a volume ratio of 3:1 to obtain 9.75g of colorless crystals, yield 88%, mp125~126℃; 1H NMR (DMSO-d 6 ): δ3.87~4.00(...
Embodiment 2
[0033] Example 2: Preparation of (E)-3,5-dimethoxy-2'-sulfonate sodium-1,2-stilbene:
[0034] Dissolve 10g (0.043mol) of 3,5-dimethoxybenzyl bromide in 14.4g (0.086mol) of triethyl phosphite, heat to 125°C, stir and reflux for 3h, distill off excess triethyl phosphite under reduced pressure The ester yielded 11.2 g of 3,5-dimethoxybenzyl phosphonate as an orange-red oil, which was directly used in the next reaction.
[0035] Add 11.2g (0.04mol) of orange-red oily 3,5-dimethoxybenzylphosphonate and 10g (0.048mol) of 2-sodium sulfonate benzaldehyde to a volume of 100ml N,N-di After stirring and dissolving in methylformamide, add dropwise 15ml of anhydrous ethanol solution of potassium tert-butoxide (0.048mol) with a content of 30% by mass, stir for 10h at room temperature, pour the reaction solution into ice water, and filter the precipitated solid , Dried, and recrystallized with a mixture of ethyl acetate and n-hexane in a volume ratio of 6:1 to obtain 10.37g of colorless crystals...
Embodiment 3
[0036] Example 3: (E) Preparation of sodium 3,5-dimethoxy-2',4'-disulfonate-1,2-stilbene:
[0037] Dissolve 10g (0.043mol) of 3,5-dimethoxybenzyl bromide in 10.7g (0.065mol) of triethyl phosphite, heat to 120°C, stir and reflux for 4 hours, distill off excess triethyl phosphite under reduced pressure The ester yielded 11.2 g of 3,5-dimethoxybenzyl phosphonate as an orange-red oil, which was directly used in the next reaction.
[0038] Add 11.2g (0.04mol) of orange-red oil 3,5-dimethoxybenzylphosphonate and 12.4g (0.04mol) of sodium 2,4-disulfonate benzaldehyde to a volume of 80ml N After stirring and dissolving in N-dimethylformamide, add 23ml of 20% by mass potassium tert-butoxide (0.04mol) in absolute ethanol solution, stir and react at room temperature for 9h, pour the reaction solution into ice water, The precipitated solid was filtered, dried, and recrystallized with a mixture of ethyl acetate and n-hexane in a volume ratio of 10:1 to obtain 11.28 g of colorless crystals, wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com