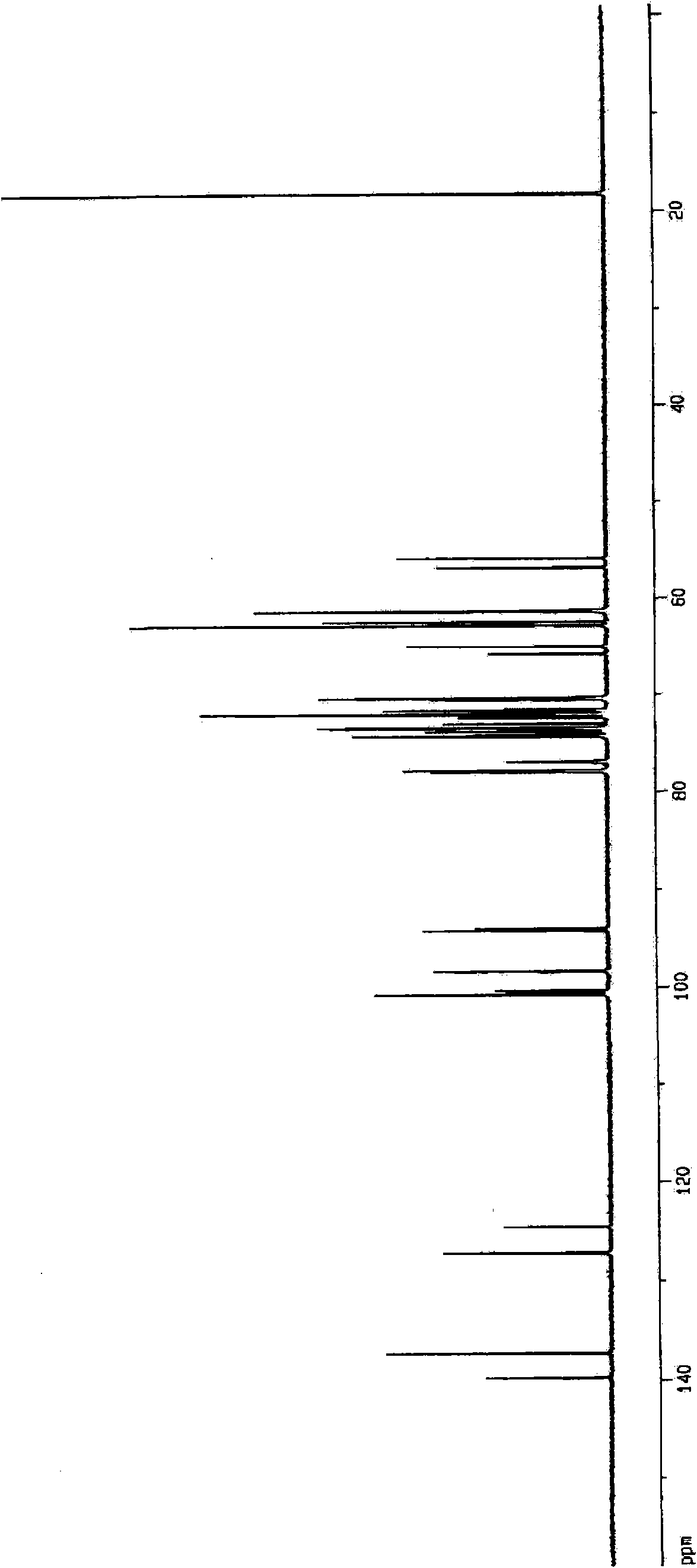
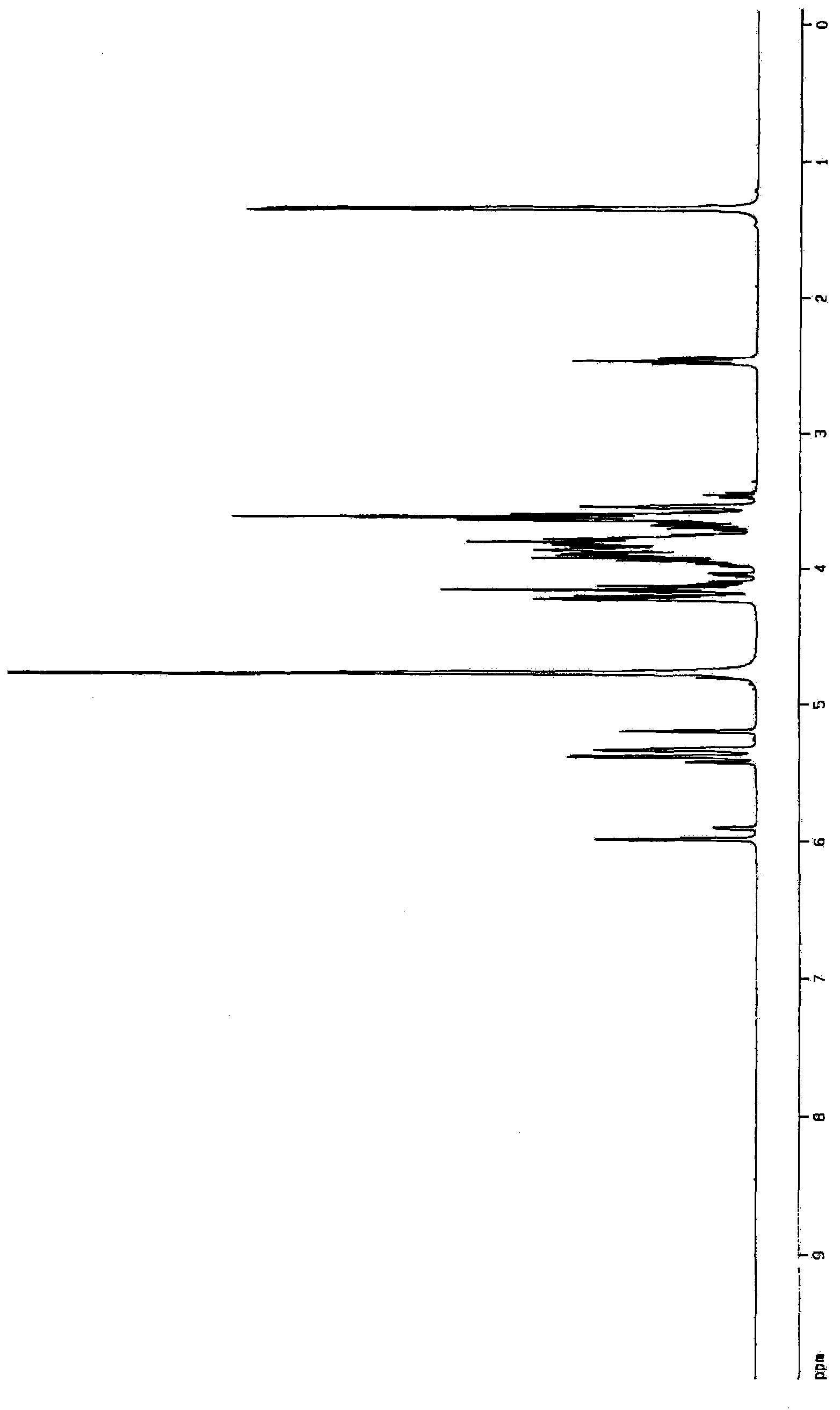
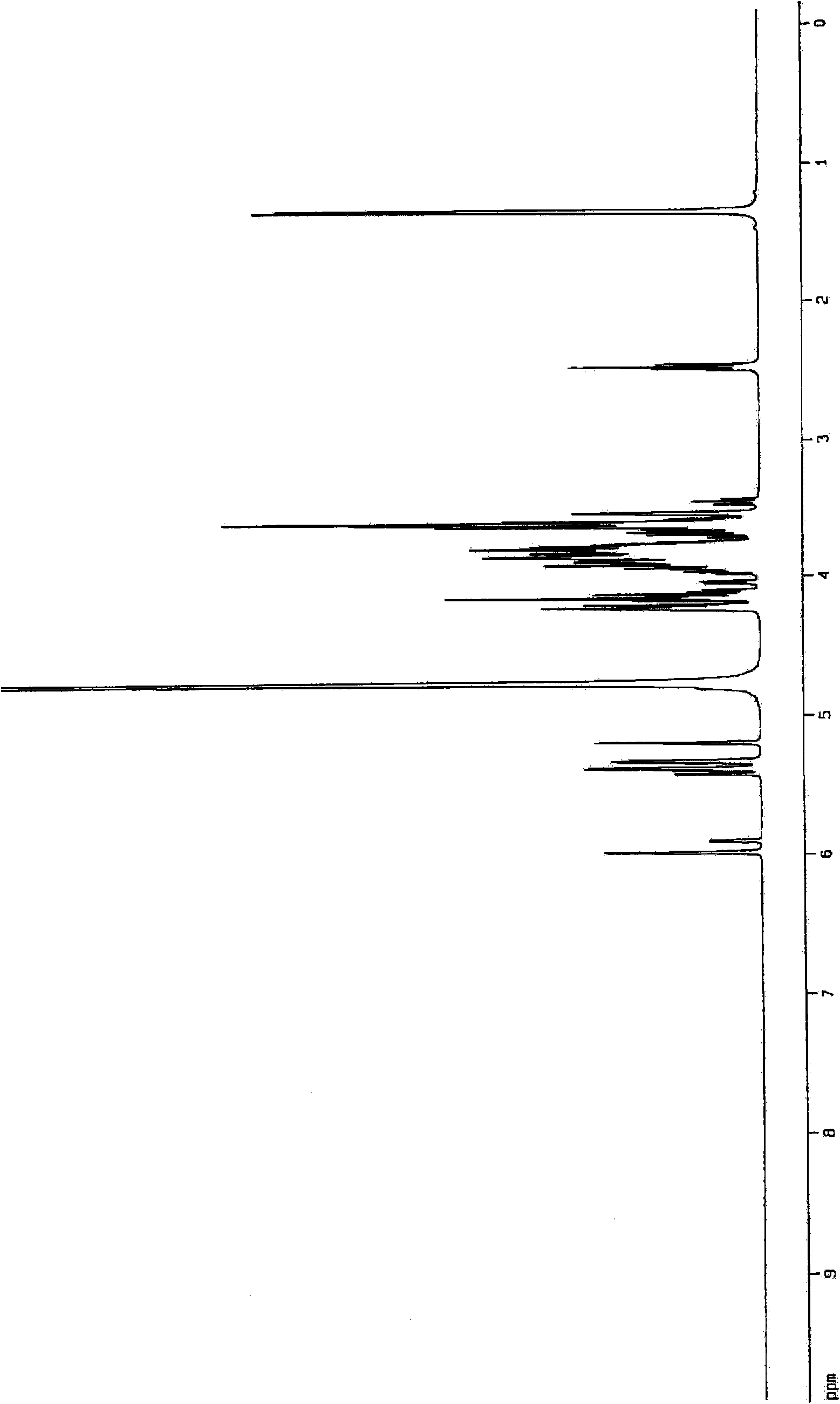Aminosugar compound and process for production thereof
A production method and compound technology are applied in the field of amino sugar compounds to achieve the effect of inhibiting sugar absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] (nourish)
[0131] In a Erlenmeyer flask with a volume of 100mL, add 30mL each time the culture medium (pH=7.0) containing 20g soluble starch, 10g Pindex #3 (manufactured by Matsutani Chemical Co., Ltd.), 20g soybean flour and 1L distilled water, at 121°C Sterilize in a pressure cooker for 30 minutes to prepare a stock culture medium.
[0132] A slant culture of the Streptomyces sp-6982 strain was inoculated into the stock culture medium by 1 platinum spoon, and cultured with shaking at 30° C. for 3 days.
[0133] Into a Erlenmeyer flask with a capacity of 100mL, 30mL each time, add 100g soluble starch, 60g soybean flour, 2.5g NaCl, 2g CaCO 3 and a culture medium (pH=7.0) of 1 L of distilled water were autoclaved at 121° C. for 30 minutes to prepare a production medium.
[0134] Into this production medium, 2 mL each time, each flask was inoculated with the above-mentioned mother seed culture solution, and cultured with shaking at 25° C. for 7 days.
[0135] Roughly ...
Embodiment 2
[0226] The α-amylase inhibitory activity of the compounds of the present invention was confirmed by the following method.
[0227] (1) Experimental method
[0228] ICR mice (male, 8 weeks old, purchased from SLC, Japan), SD rats (male, 8 weeks old, purchased from Japan’s Chayaru Srivier), beagle dogs (male, 35 months old, purchased from Naruku Co., Ltd.) and rhesus monkey (male, 10 years old, purchased from Japan CLEA) to prepare mouse, rat, dog and monkey pancreas α-amylase solutions. Human salivary and pancreatic alpha-amylases were prepared from enzymes purchased from Sigma-Aldrich. These α-amylase solutions were all assayed with buffer (48mM NaCl, 5.4mM KCl, 28mM Na 2 HPO 4 , 43mMNaH 2 PO 4 , 35mM mannitol, pH=7.0) diluted to 800U / mL. Various α-amylase solutions (20 U, 25 μL) and compounds (25 μL) dissolved in assay buffer were added to 96-well microplates and incubated at 37° C. for 10 minutes. Then starch solution (5 mg / mL, 50 μL) was added and incubated at 37° C. f...
Embodiment 3
[0232] The oral activity of the compound of the present invention was confirmed by the following method.
[0233] (1) Experimental method
[0234] As animals, male ICR (normal) mice (6 weeks old, purchased from Japan SLC) were used. Compounds were made into solutions with 0.5% methylcellulose solution. Blood was collected from mice fasted overnight for determination of blood glucose and plasma insulin levels, and immediately after oral administration of solvent or compound A (0.3 mg / kg, 1 mg / kg, 3 mg / kg, 10 mg / kg) Carbohydrate solution (75 mg / mL starch, 25 mg / mL sucrose, 20 mL / kg). Next, blood was collected 0.25 hours, 0.5 hours, and 1 hour later for measuring plasma insulin levels, and blood was collected 0.5 hours, 1 hour, and 2 hours later for measuring blood sugar levels.
[0235] Blood glucose levels were measured using Glucos CII-Testowako reagent (manufactured by Wako Pure Chemical Industries, Ltd.), and plasma insulin concentrations were measured using a mouse insul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com