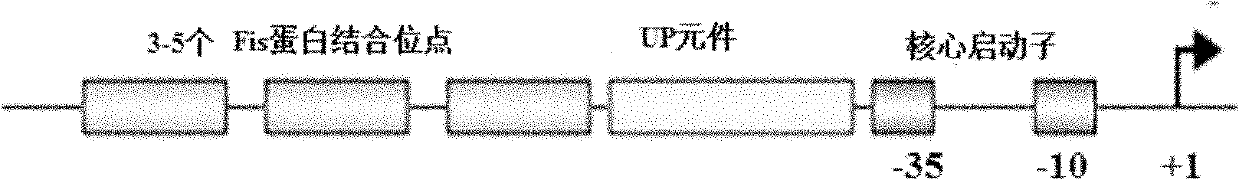
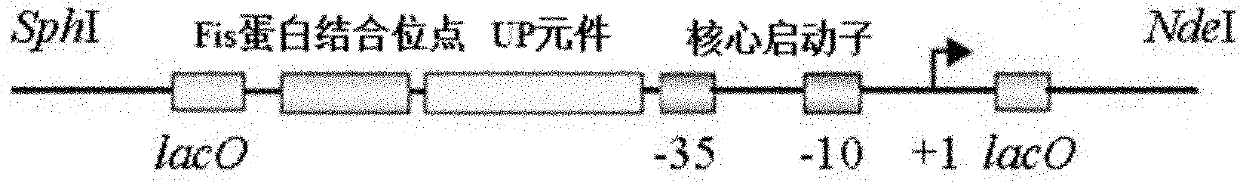
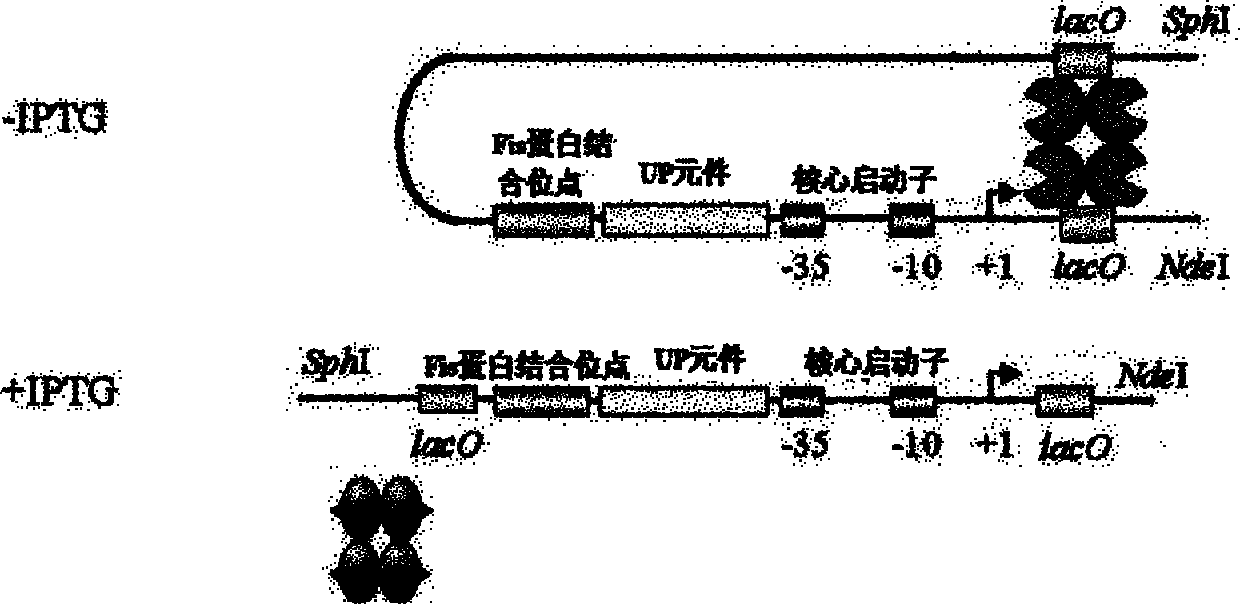
RRNA mosaic promoter and expression vector containing same
A technology of chimeric promoters and expression vectors, applied in the field of molecular biology, can solve problems such as difficult regulation and achieve high-efficiency expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Promoter Fragment P 16S-2 Amplification and construction of intermediate plasmid p16S-2
[0044] according to E. coli rRNA rrnB P1 promoter sequence and lac operon lacO Sequence design PCR primers, specifically:
[0045] Upstream primer S1: SEQ ID NO. 3 (containing Sph I restriction site), downstream primer S2: SEQ ID NO. 4. P 16S-2 Promoter fragment. The PCR conditions are: 10 μL 5×PrimeSTAR Buffer (Mg 2+ plus), 50 μM dNTP Mixture, 0.5 μM S1, 0.5 μM S2, 1.25 U PrimeSTAR HS DNA Polymerase (TAKARA), and adjust the reaction volume to 50 μL with sterile water. The PCR amplification reaction program was: 98 °C, 10 s, 55 °C, 15 s, 72 °C, 30 s, 29 cycles; 72 °C, 10 min.
[0046] PCR products were purified by 2% agarose gel electrophoresis, and Taq The enzyme can add deoxyadenosine to the end of the amplified fragment, and PCR is performed again to fill in the sticky end of each fragment and add a dA. The PCR conditions are: 2.5 μL 10× Taq Buf...
Embodiment 2
[0051] Embodiment 2: Construction of expression vector pRNP2
[0052] The p16S-2 and pET29a plasmids were double-digested with NdeI and SphI to obtain the promoter fragment P16S-2 and the double-digested fragment of pET29a with the T7 promoter removed. After purification and recovery, they were mixed with enzymes at a ratio of 2:1. The enzyme-linked product was transformed into E.coli DH10B competent cells, and LB plates containing 50 μg·mL-1 kanamycin were spread. The transformant was identified by PCR and sequencing to obtain the expression vector pRNP2.
Embodiment 3
[0053] Example 3: Construction of recombinant plasmid pRNP2-cel5G and expression strain containing β-1,4 endoglucanase gene (cel5G)
[0054] pRNP2 and the recombinant plasmid pET29a- cel5 G (Cui Zhongli et al., Chinese patent application number: 201010018298.0) used respectively xho I and Nde I perform double digestion. The enzyme digestion system is:
[0055] Nde I enzyme (10 U·μL -1 )
1.0 μL
xho I enzyme (10 U·μL -1 )
1.0 μL
10× universal buffer
5.0 μL
pET29a- cel5 G
20.0 μL
double distilled water
24.0 μL
total capacity
50.0 μL
[0056] After reacting in a water bath at 37 °C for more than 3 h, the digested products were recovered by 0.75% agarose gel electrophoresis, and the method was the same as above.
[0057] The digested products were separated and purified by agarose gel electrophoresis, and then recovered with a DNA gel recovery kit. Then the double digested cel5 T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com