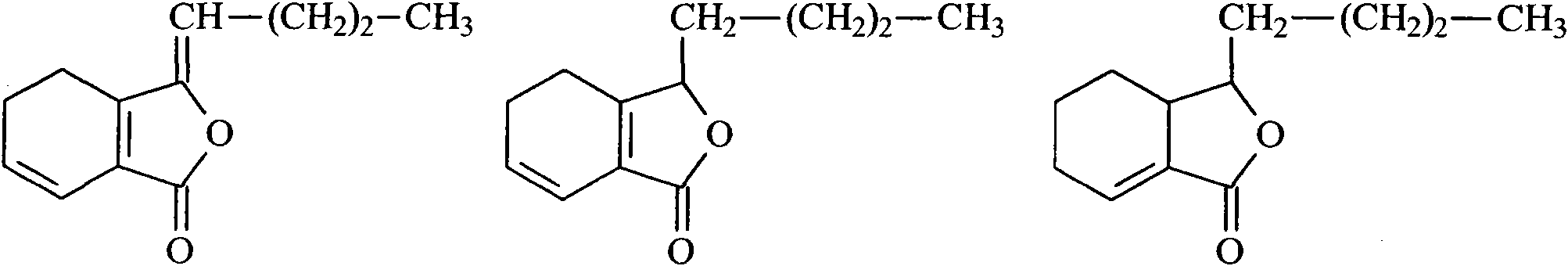
Szechuan lovage rhizome total phthalide extract
A technology for total phthalides and extracts, applied in the field of total phthalides of Chuanxiong Rhizoma Rhizoma Chuanxiong and its preparation, can solve the problems of undefined technical quality standards, strict standards for raw material selection, and large differences in pharmacological activity. , to achieve significant pharmacological activity, significant curative effect, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
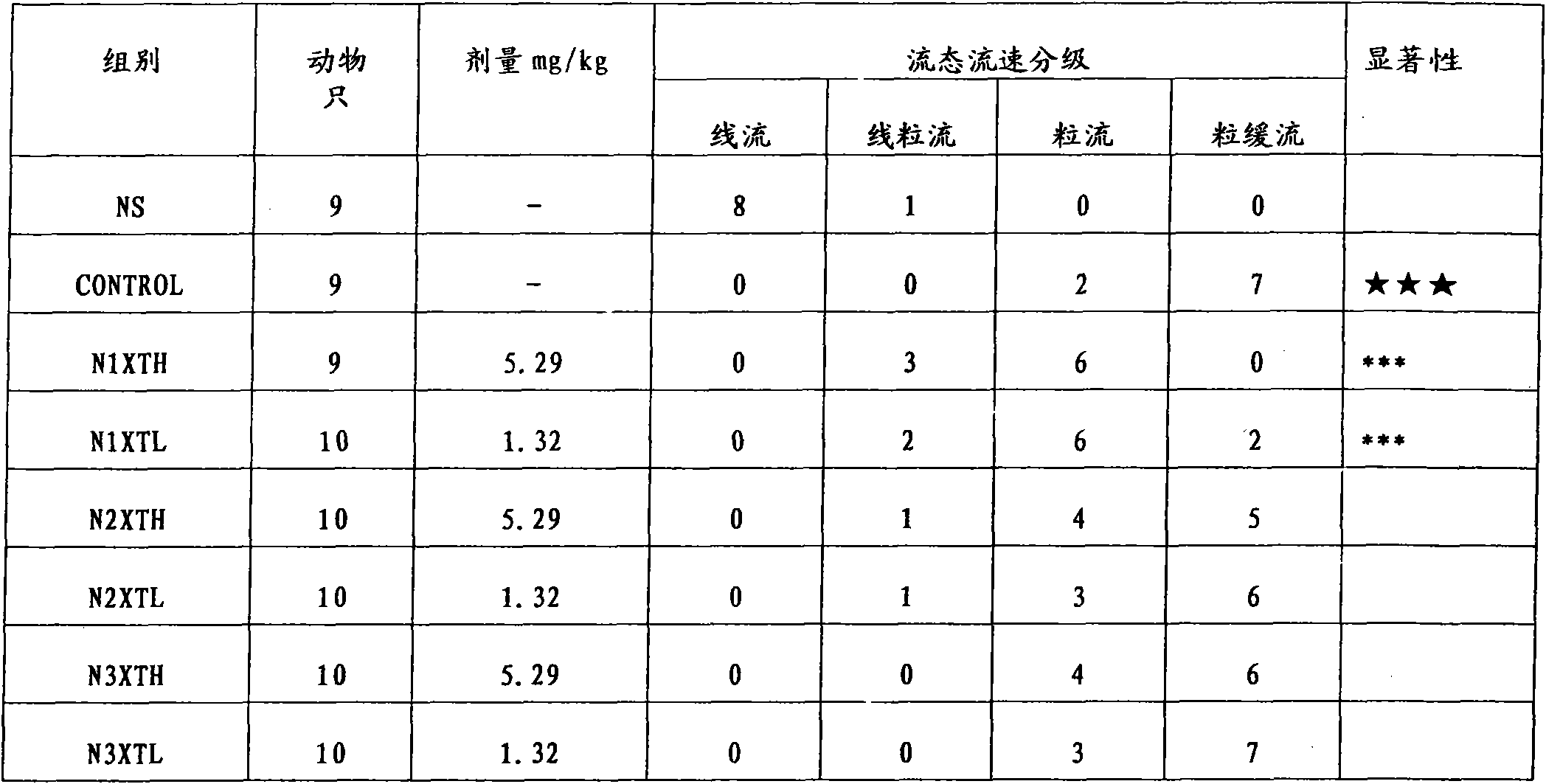
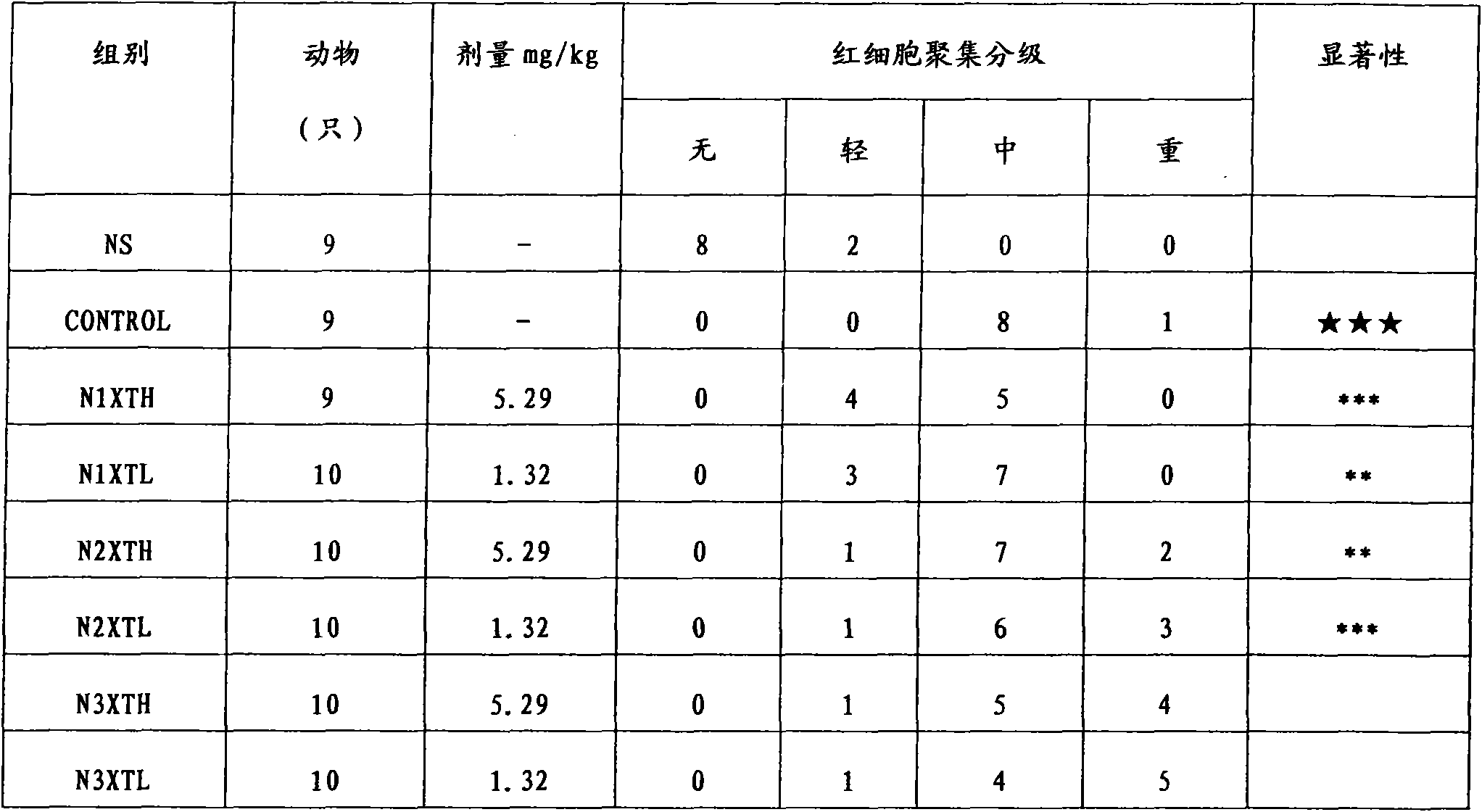
[0035]After the Rhizoma Chuanxiong medicinal material was removed from impurities, washed and dried, 100kg was taken and pulverized into fine powder. The Rhizoma Chuanxiong fine powder was measured by high-performance liquid chromatography, and the main component ligustilide (ligustilide) accounted for 1.61% of the original medicinal material; n-butyl- The total content of 4,5-dihydrophthalide (Senkyunolide A) and neocnidilide (neocnidilide) is measured by the external standard of n-butyl-4,5-dihydrophthalide (Senkyunolide A), accounting for 0.64% of the original medicinal material; The ratio of ligustilide to the sum of n-butyl-4,5-dihydrophthalide (Senkyunolide A) and neocnidilide (neocnidilide) is 2.5:1. Conform to the relevant requirements of feeding. Wet the Rhizoma Chuanxiong fine powder with 100kg of 85% ethanol, then evenly add it into the percolation tank, add 2 times the weight of 200kg of ethanol at the same concentration, soak for 1 hour, and drip at a rate of 1.5m...
Embodiment 2
[0037] After removing impurities, washing and drying the Rhizoma Chuanxiong medicinal material, take 100kg and pulverize it into a fine powder. The Ligusticum Rhizoma Chuanxiong fine powder is determined by high performance liquid chromatography, and the main component ligustilide accounts for 1.35% of the original medicinal material; n-butyl-4,5 - The total content of dihydrophthalide and neocnilide is measured by n-butyl-4,5-dihydrophthalide external standard, accounting for 0.56% of the original medicinal material; ligustilide and n-butyl-4,5-dihydrophthalide The ratio of the sum of hydrophthalide and neocnilide is 2.41:1. Conform to the relevant requirements of feeding. Wet the Rhizoma Chuanxiong fine powder with 100kg of 90% ethanol, which is 1 times the weight, and then evenly add it to the percolation tank, then add 2 times the weight, 200kg, of the same concentration of ethanol, soak for 1 hour, and drip at a rate of 1.5ml / kg min The seepage liquid is collected, and t...
Embodiment 3
[0039] After removing impurities, washing and drying the Rhizoma Chuanxiong medicinal material, take 100kg and grind it into a fine powder. The fine powder of Rhizoma Chuanxiong is determined by high performance liquid chromatography, and the main component ligustilide accounts for 0.28% of the original medicinal material; n-butyl-4,5 - The total content of dihydrophthalide and neocnilide is measured by n-butyl-4,5-dihydrophthalide external standard, accounting for 0.12% of the original medicinal material; ligustilide and n-butyl-4,5-dihydrophthalide The ratio of the sum of hydrophthalide and neocnilide is 2.33:1. Does not meet the relevant requirements for feeding. Wet the Rhizoma Chuanxiong fine powder with 100kg of 85% ethanol, then evenly add it into the percolation tank, add 2 times the weight of 200kg of ethanol at the same concentration, soak for 1 hour, and drip at a rate of 1.5ml / kg min The seepage liquid is collected, and the seepage liquid is concentrated under red...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com