Method for preparing polymeric aluminum ferric sulfate by using aluminium ore washing gangue
A technology for polymerizing ferric aluminum sulfate and aluminum sulfate, which is applied in the fields of ferric sulfate, flocculation/precipitation water/sewage treatment, etc. It can solve the problems of low degree of polymerization, low product yield, and source limitation, and achieve reduced production costs and efficient circulation Economical and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
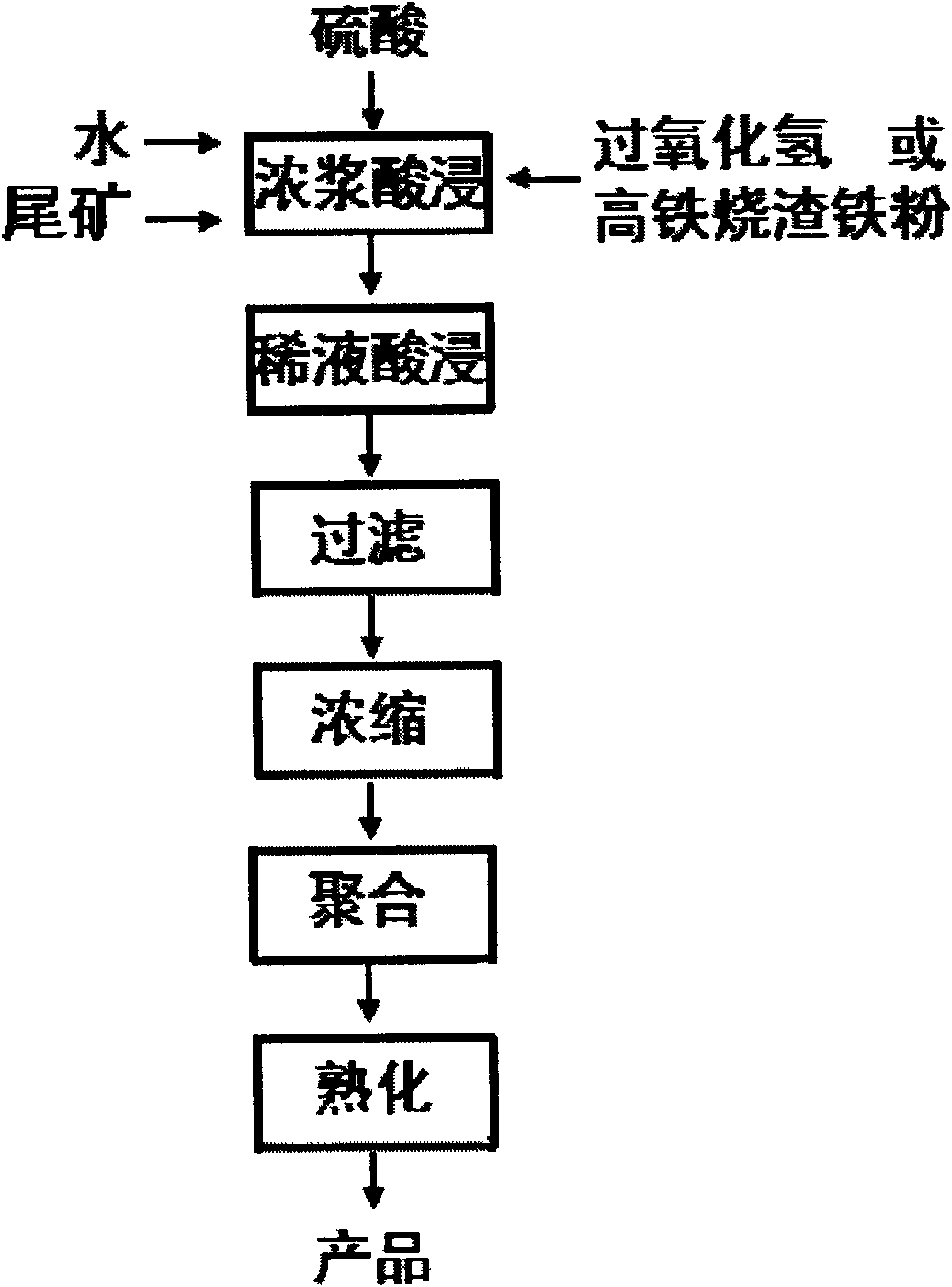
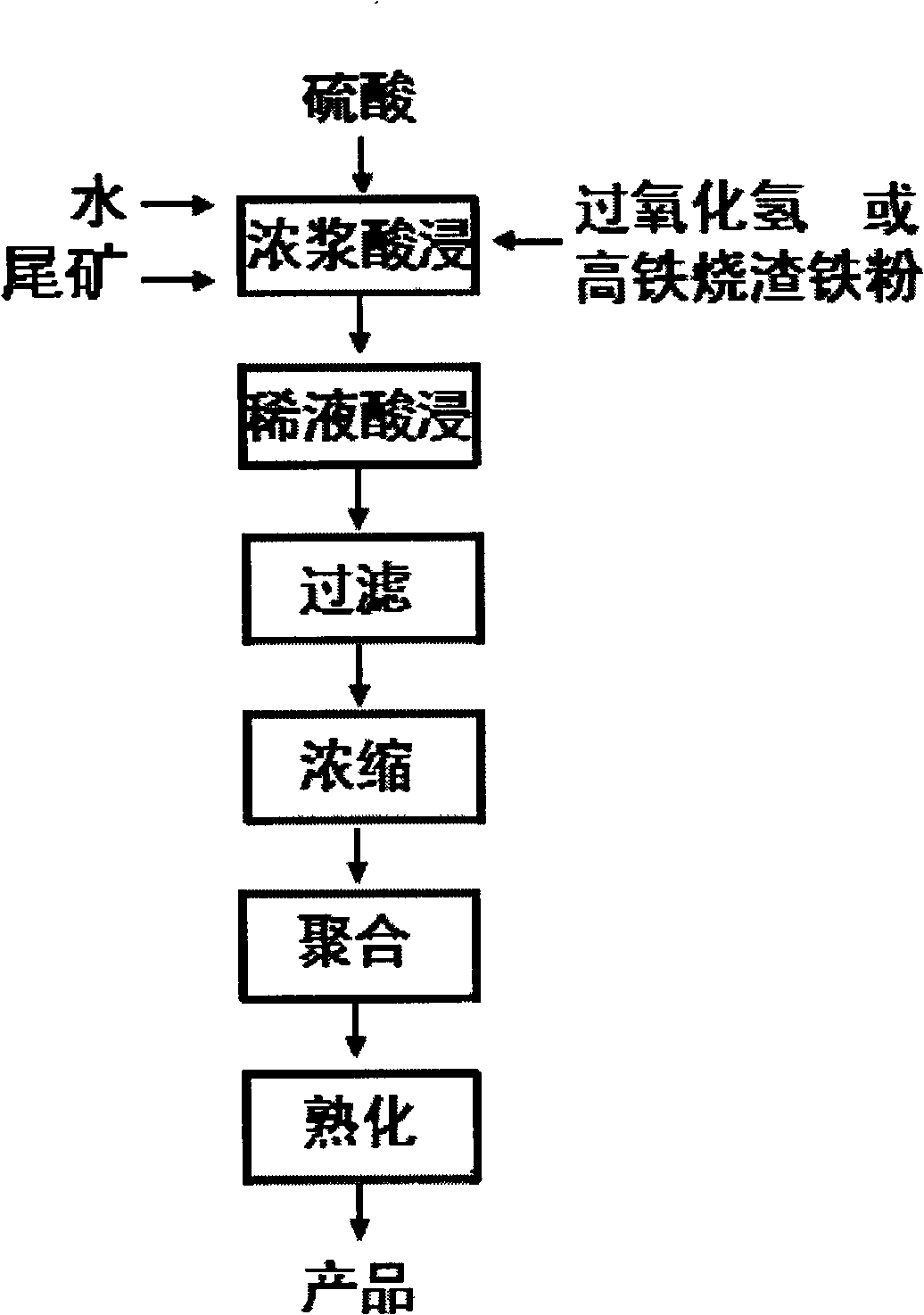
[0049] First, add water, tailings, sulfuric acid and hydrogen peroxide in a ratio of 3.7:1:1.85:0.025 by weight of raw materials into the reaction kettle, heat to 150°C, start stirring to carry out thick slurry acid leaching reaction, thick slurry acid leaching Time 41 minutes, control Al 2 o 3 The leaching rate is 72%, Fe 2 o 3 The leaching rate is 90%; 3 parts by weight of water is added to the acid leaching solution, and the acid leaching reaction is continued for 4 hours after cooling down to 80°C. After the acid leaching reaction is finished, use a ceramic filter to filter to obtain an aqueous solution of aluminum sulfate and ferric sulfate, and the filter residue is used as a cement filler; the aqueous solution of aluminum sulfate and ferric sulfate is concentrated, and when the liquid concentration reaches 40 degrees Baume, the following The weight ratio is: leaching solution: sodium bicarbonate = 1: 0.15 alkalizing agent for polymerization, the polymerization stirring...
Embodiment 2
[0051] First add water, ore slime (tailings), sulfuric acid and hydrogen peroxide in a ratio of 3.7:1:1.85:0.025 by weight of raw materials into the reaction kettle, heat to 148° C., start stirring to carry out thick slurry acid leaching reaction, Thick pulp acid leaching time is 40 minutes, control Al 2 o 3 The leaching rate is 75%, Fe 2 o 3 The leaching rate was 92%; 3.2 parts by weight of water was added to the acid leaching solution, and the acid leaching reaction was continued for 4 hours after cooling down to 79°C. After the acid leaching reaction is finished, use a ceramic filter to filter to obtain an aqueous solution of aluminum sulfate and ferric sulfate, and the filter residue is used as a cement filler; the aqueous solution of aluminum sulfate and ferric sulfate is concentrated, and when the liquid concentration reaches 40 degrees Baume, the following The weight ratio is: leaching solution: sodium carbonate = 1: 0.15 alkalizing agent for polymerization, the poly...
Embodiment 3
[0053] First add water, ore slime (tailings), sulfuric acid and hydrogen peroxide in a ratio of 3.7:1:2.78:0.025 by weight of raw materials into the reaction kettle, heat to 146°C, start stirring to carry out thick slurry acid leaching reaction, After 20 minutes of acid leaching reaction, add: 1.5 parts by weight of high iron slag and iron powder and heat to 150°C to continue the reaction for 22 minutes to control Al 2 o 3 The leaching rate is 75%, Fe 2 o 3 The leaching rate was 94%; 3.5 parts by weight of water was added to the acid leaching solution, and the acid leaching reaction was continued for 4 hours after cooling down to 80°C. After the acid leaching reaction is finished, use a ceramic filter to filter to obtain an aqueous solution of aluminum sulfate and ferric sulfate, and the filter residue is used as a cement filler; the aqueous solution of aluminum sulfate and ferric sulfate is concentrated, and when the liquid concentration reaches 40 degrees Baume, the follow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com