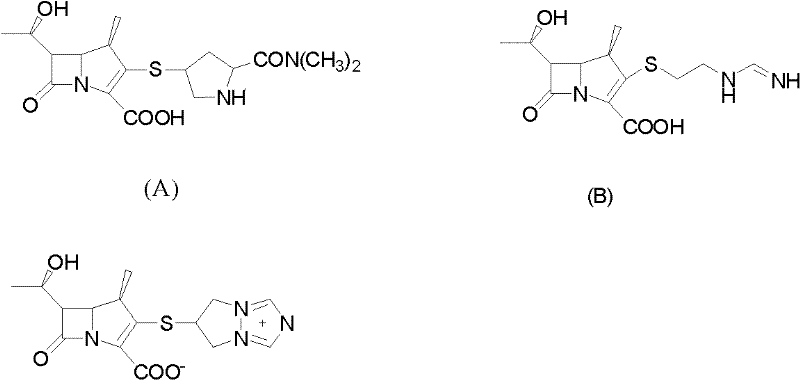
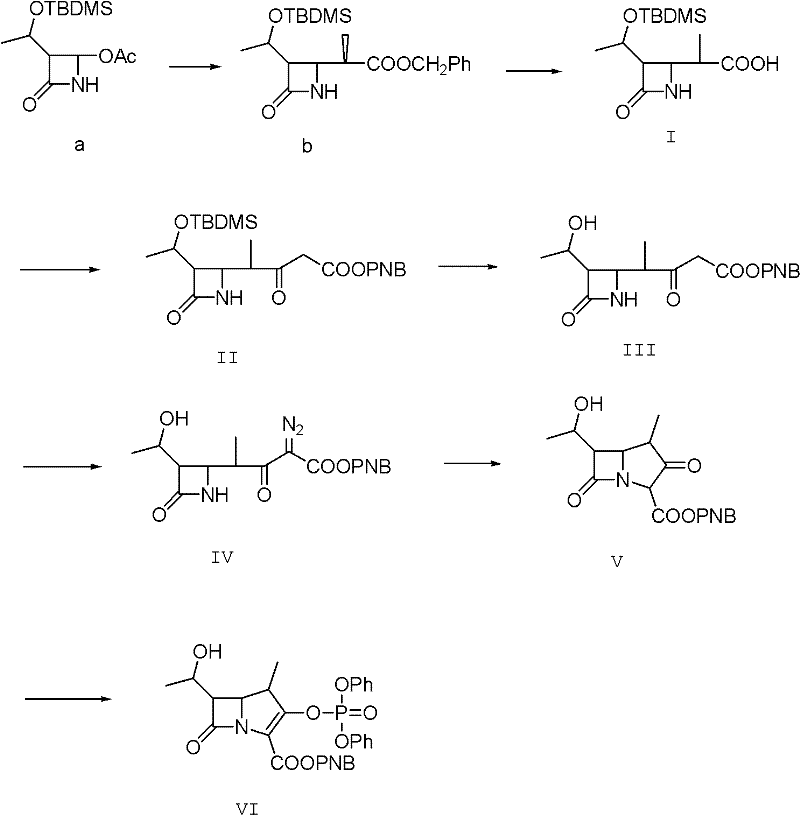
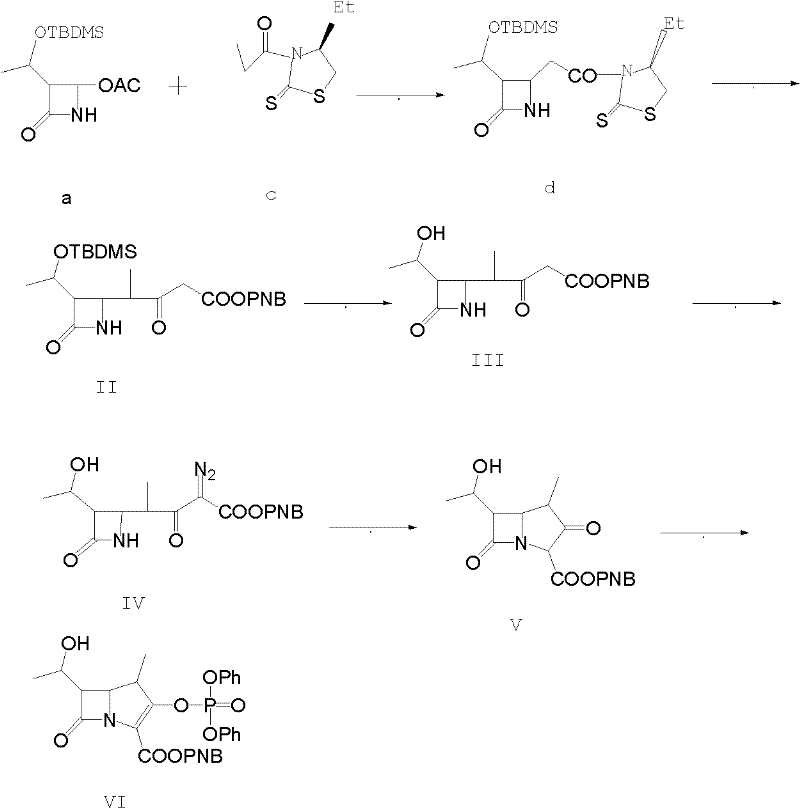
Method for preparing protected meropenem
A technology for meropenem and a compound, which is applied in the field of synthesizers in the field of medical technology, can solve the problems of low yield of meropenem, unfavorable sustainable development, and difficulty in obtaining raw material c, and achieves high molar yield, low cost, and high production efficiency. Easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1, compound (II) is also (3S, 4R)-3-[(1R)-1-tert-butyldimethylsiloxyethyl]-4[(1R)-1-methyl-3- Synthesis of PNB oxycarbonyl-2-ketopropyl]-azetidin-2-one:
[0034] 50g (3S, 4R-3-[(1R)-1-tert-butyldimethylsiloxyethyl]-4-[(1R)-1-methyl-1-carboxyethyl]-azacycline Butan-2-one, that is, compound (I), was added to 750ml of dichloromethane, and 34g of carbonyldiimidazole was added under stirring, and stirred at room temperature for 30min, and then 16.0g of anhydrous magnesium chloride, 34.0g of triethylamine, p-nitrogen 70g of benzyl alcohol malonate monoester, after the feeding is completed, the temperature is raised to 40-43°C, and the reaction is refluxed for 6.0h. After the reaction is completed by HPLC tracking, compound (II) is generated. After the reaction is completed, cool to 15-20°C, and add dropwise 1200g of 5% hydrochloric acid, temperature control 15-20°C, stirring and static layering after the dropwise addition, the organic phase is washed with salt water ...
Embodiment 2
[0035] Example 2, compound (III) is (3S,4R)-3-[(1R)-1-tert-butyldimethylsiloxyethyl]-4-[(1R)-1-methyl-3 - Synthesis of diazo-3-PNBoxycarbonyl-2-ketopropyl]-azetidin-2-one.
[0036] Add 200ml of water to the flask, add 15.0g of sodium azide, stir to dissolve, add dropwise 25.0g of methanesulfonyl chloride at 15-20°C for about 30 minutes, add 200ml of acetone to make it, and control the temperature for 15-20°C Stir and react at 20°C for 2.0h, then cool down to 0-5°C and add compound (II) acetone solution of Example 1, stir and react at 0-5°C for 8.0h, after the reaction is complete, add 250ml of water dropwise within 30 minutes. After the addition, keep stirring at about 5°C for 1 hour; centrifuge and dry to obtain about 102 g of compound (III) as a light yellow to orange solid, which is directly fed downward.
Embodiment 3
[0037] Example 3, compound (IV) is (3S, 4R)-3-[(1R)-1-hydroxyethyl]-4-[(1R)-1-methyl-3-diazo-3-PNB Synthesis of oxycarbonyl-2-ketopropylbuta]-azetidin-2-one.
[0038] The compound (III) obtained in Example 2 was dissolved in methanol, and then 21.0 g of methanesulfonic acid was added dropwise, the temperature was controlled at 20-25° C., and the reaction was carried out under stirring for 8.0 h. After the completion of the reaction was followed by HPLC, compound (VI) was generated. After recovering methanol under reduced pressure, add 600ml of ethyl acetate and stir to dissolve, wash the organic phase with water, then wash with saturated brine, then wash with 100ml of 1.5% sodium bicarbonate by mass percentage, combine the organic phases, add activated carbon and anhydrous magnesium sulfate for decolorization , dry, filter, and then reclaim the extraction solvent under reduced pressure to dryness, add 120ml of ethyl acetate to dissolve, stir and dissolve at 15-20°C, add 1g of se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com