Method for solubilizing carbon nano tube by using super-molecular complex and controlling solubility thereof by using light
A technology of supramolecular composites and carbon nanotubes, which is applied in the direction of non-effective components of polymer compounds, chemical instruments and methods, medical preparations of non-effective components, etc., to achieve the effects of increasing application prospects, simple operation, and reducing cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
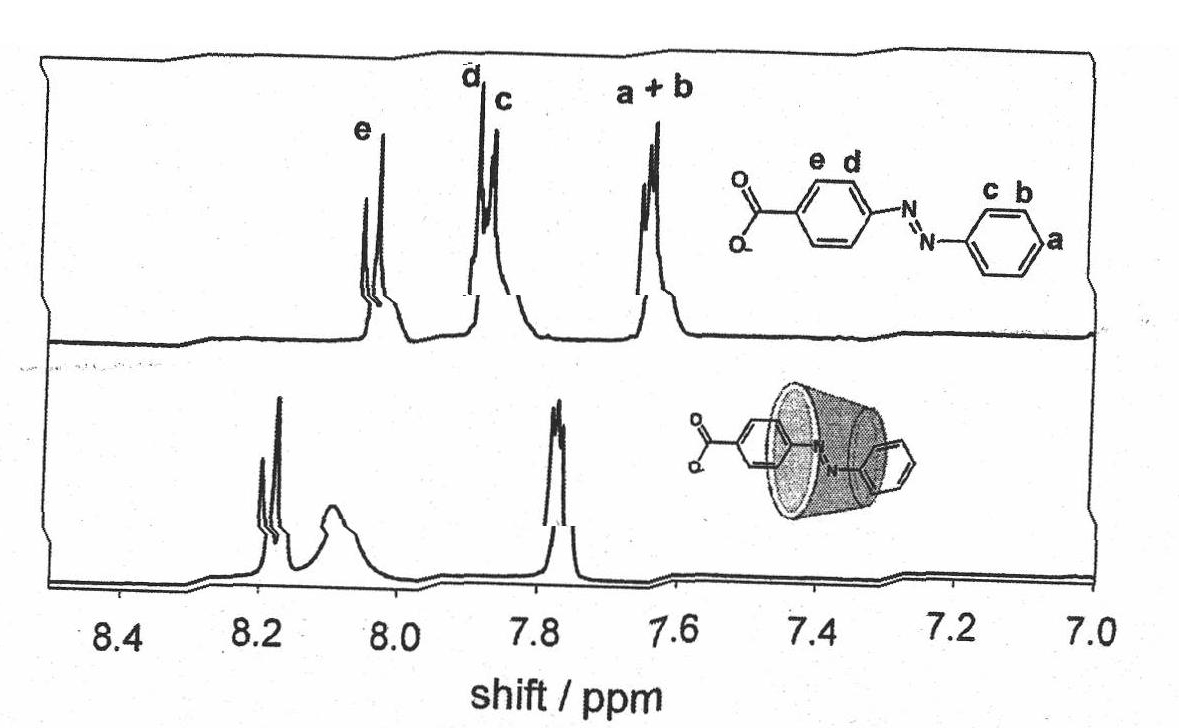
[0034] Example 1. The supramolecular complex formed by sodium azobenzoate and α-cyclodextrin solubilizes multi-walled carbon nanotubes
[0035]Step 1: Preparation of Sodium Azobenzoate
[0036]
[0037] (1) Catalyst MoO 5 ·H 2 Preparation of O·HMPA
[0038] In a 100mL three-neck flask, add 5g MoO 3 and 35 mL of 30% H 2 o 2 Aqueous solution, stirred at 40°C for 4 hours, lowered to room temperature, filtered with suction to obtain a yellow filtrate, added 5.0 mL of hexamethylphosphoric triamide (HMPA) in an ice bath; filtered with suction under reduced pressure; recrystallized with methanol to obtain 3.0 g MoO 5 ·H 2 O.HMPA product in 29% yield.
[0039] (2) Preparation of ethyl p-nitrosobenzoate
[0040] Weigh 10g ethyl 4-aminobenzoate, 2.4g MoO 5 ·H 2 O·HMPA was dissolved in 100mL of dichloromethane, and then 40mL of 30% H 2 o 2 aqueous solution, stirred and reacted at 30°C for 20 hours. After the reaction, wash with water, extract the reaction solution with 2...
Embodiment 2
[0046] Example 2, the supramolecular complex formed by sodium azobenzoate and β-cyclodextrin solubilizes multi-walled carbon nanotubes
[0047] The steps are the same as in Example 1, the only difference is that in step 2, β-cyclodextrin is used instead of α-cyclodextrin, and the solubility of carbon nanotubes is shown in Table 1.
[0048] Table 1. Solubility of carbon nanotubes in aqueous solutions of different concentrations of sodium azobenzoate and supramolecular complexes formed with α- and β-cyclodextrin
[0049]
Embodiment 3
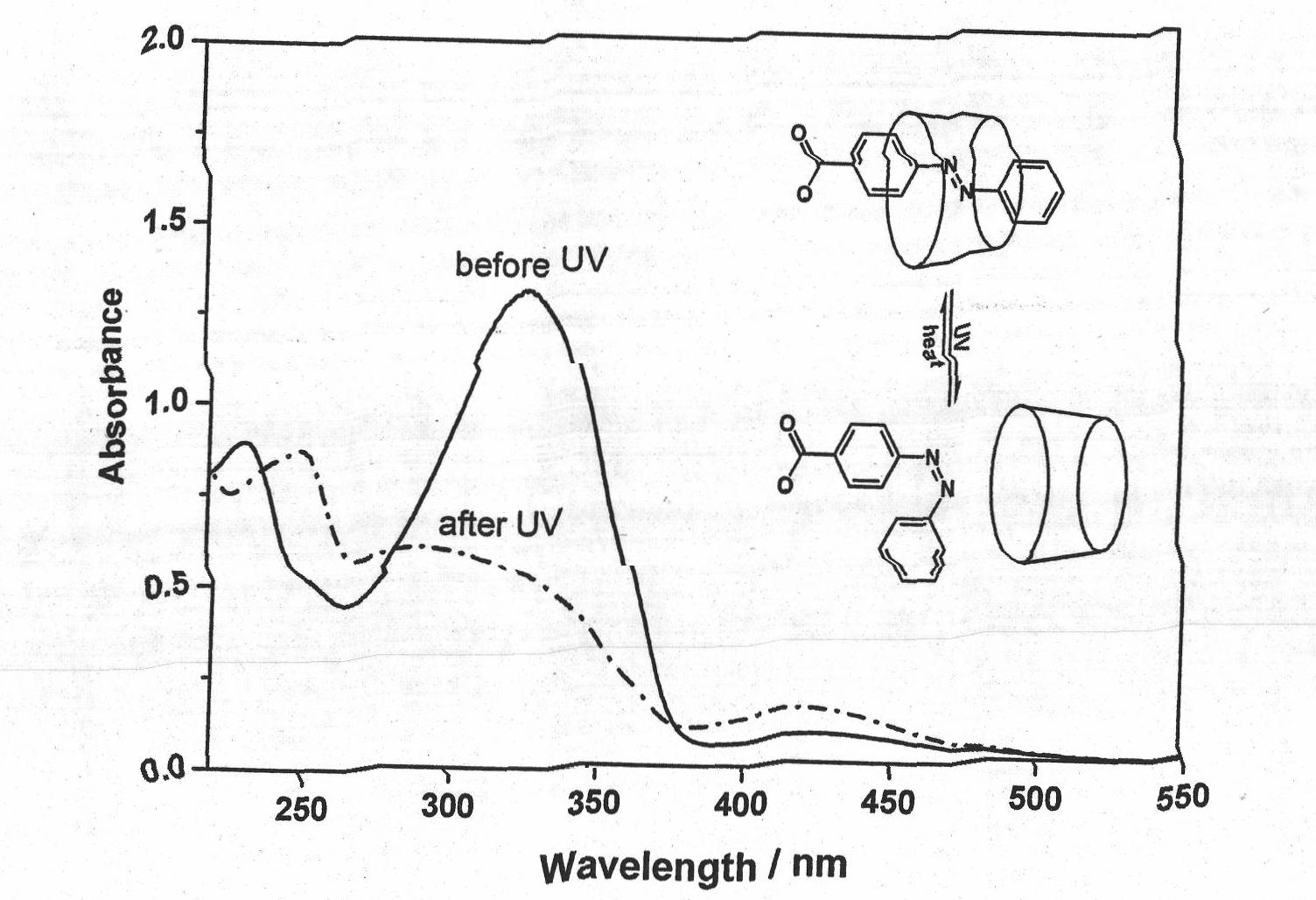
[0050] Embodiment 3, using light to control the solubility of multi-walled carbon nanotubes
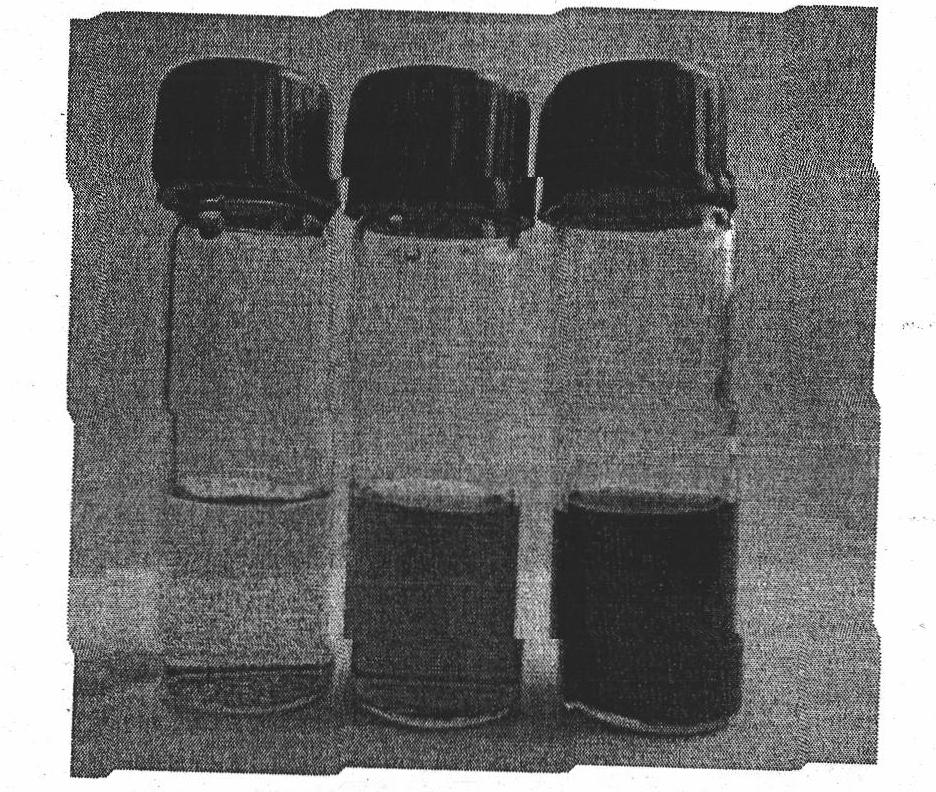
[0051] The preparation concentration is 1×10 -4 Add an equimolar amount of cyclodextrin to 10 mL of a mol / L sodium azobenzoate aqueous solution, and sonicate for 5 minutes to form a supramolecular complex aqueous solution. Weigh 3 mg of multi-walled carbon nanotubes and place them in a centrifuge tube, then add 10 mL of supramolecular complex aqueous solution, and mix. Sonicate at 20°C for 5 hours, and then divide the solution evenly into two parts, A and B. The solution A was irradiated with a 325nm laser for 45 minutes, the solution B was allowed to stand in the dark for 45 minutes, and then the solutions A and B were simultaneously centrifuged for 30 minutes (8000 rpm).
[0052] Table 2 lists the influence of light on the solubility of carbon nanotubes in the presence of no cyclodextrin and the presence of α- or β-cyclodextrin. It can be seen from the table that light will reduc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com